The stoichiometric reaction of 516 g of dimethyldichlorosilane with water results in a tetrameric cyclic product X in 75% yield. The weight (in g) of X obtained is___ .
[Use, molar mass (g mol−1): H = 1, C = 12, O = 16, Si = 28, Cl = 35.5]
[Use, molar mass (g mol−1): H = 1, C = 12, O = 16, Si = 28, Cl = 35.5]
Correct Answer: 222
Solution and Explanation

Number of moles of Dimethyldichlorosilane = \(\frac{516}{129}=4\) mole

We can apply POAC on the Si atom.
Moles of tetrameric formed = \(\frac{4}{4}\times\frac{75}{100}=0.75\) moles
The molar mass of the product formed = \(296\times0.75=222\,g\)
So, the answer is \(222\).
Top Questions on Ideal gas equation
- The stoichiometric reaction of 516 g of dimethyldichlorosilane with water results in a tetrameric cyclic product X in 75% yield. The weight (in g) of X obtained is___. [Use, molar mass (g mol−1): H = 1, C = 12, O = 16, Si = 28, Cl = 35.5]
- JEE Advanced - 2023
- Chemistry
- Ideal gas equation
- The total pressure of a mixture of non-reacting gases $X (0.6 \,g )$ and $Y (0.45 \, g )$ in a vessel is $740 mm$ of $Hg$ The partial pressure of the gas $X$ is ____$mm$ of $Hg$(Nearest Integer)(Given : molar mass $X =20$ and $Y =45 \, g \, mol ^{-1}$ )
- JEE Main - 2023
- Chemistry
- Ideal gas equation
- A certain quantity of real gas occupies a volume of 30.15 dm3 at 100 atm and 500 K when its compressibility factor is 1.07. Its volume at 300 atm and 300K (When its compressibility factor is 1.4) is _____ x 10-4 dm3 (Nearest integer)
- JEE Main - 2023
- Chemistry
- Ideal gas equation
A sealed flask with a capacity of $2\, dm ^3$ contains $11 \, g$ of propane gas The flask is so weak that it will burst if the pressure becomes $2\, MPa$ The minimum temperature at which the flask will burst is ______${ }^{\circ} C$ [Nearest integer]
(Given: $R =8.3 \,J \,K ^{-1} mol ^{-1}$ Atomic masses of $C$ and $H$ are $12\, u$ and $1 \,u$ respectively) (Assume that propane behaves as an ideal gas)
- JEE Main - 2023
- Chemistry
- Ideal gas equation
- How could you increase the extent of adsorption (gas adsorbed per unit mass of solid) of a gas on a solid surface in case of physisorption ?
- WBJEE JENPAS UG - 2023
- Chemistry
- Ideal gas equation
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
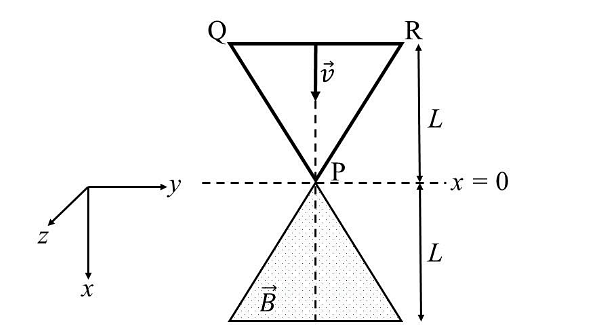
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
Concepts Used:
Ideal Gas Equation
An ideal gas is a theoretical gas composed of a set of randomly-moving point particles that interact only through elastic collisions.
What is Ideal Gas Law?
The ideal gas law states that the product of the pressure and the volume of one gram molecule of an ideal gas is equal to the product of the absolute temperature of the gas and the universal gas constant.
PV=nRT
where,
P is the pressure
V is the volume
n is the amount of substance
R is the ideal gas constant
Ideal Gas Law Units
When we use the gas constant R = 8.31 J/K.mol, then we have to plug in the pressure P in the units of pascals Pa, volume in the units of m3 and the temperature T in the units of kelvin K.



