The major product formed in the following reaction is
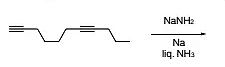
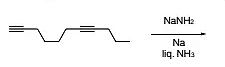
The Correct Option is B
Approach Solution - 1

It is a case of Birch reduction. Alkynes on reaction with alkali metal in liq. NH3 gives trans-alkene. But terminal alkynes do not get reduced.
The correct answer is option (B):
Approach Solution -2

Option (B) states that alkynes undergo Birch reduction in liquid ammonia to form trans-alkenes, but terminal alkynes do not undergo this reduction. This succinctly summarizes the process known as Birch reduction, where alkynes react with alkali metals in liquid ammonia to produce trans-alkenes, except for terminal alkynes, which remain unaffected by this reaction. Therefore, option (B) accurately describes the characteristic behavior of alkynes under Birch reduction conditions.
Top Questions on introduction to organic chemistry
The number of σ bonds, π bonds and having pair of electrons in pyridine respectively.
- NEET (UG) - 2023
- Chemistry
- introduction to organic chemistry
- Increasing order of stability of the resonance structures is:

Choose the correct answer from the options given below:- JEE Main - 2023
- Chemistry
- introduction to organic chemistry
- The number of colloidal systems from the following, which will have 'liquid' as the dispersion medium, is____.
Gem stones, paints, smoke, cheese, milk, hair cream, insecticide sprays, froth, soap lather.- JEE Main - 2023
- Chemistry
- introduction to organic chemistry
- Weight (g) of two moles of the organic compound, which is obtained by heating sodium ethanoate with sodium hydroxide in the presence of calcium oxide is :
- NEET (UG) - 2023
- Chemistry
- introduction to organic chemistry
R is one of the monomers for the formation of a polymer called
- TS EAMCET - 2023
- Chemistry
- introduction to organic chemistry
Questions Asked in JEE Advanced exam
- Let the function \(f:[1,\infin)→\R\) be defined by
\(f(t) = \begin{cases} (-1)^{n+1}2, & \text{if } t=2n-1,n\in\N, \\ \frac{(2n+1-t)}{2}f(2n-1)+\frac{(t-(2n-1))}{2}f(2n+1) & \text{if } 2n-1<t<2n+1,n\in\N. \end{cases}\)
Define \(g(x)=\int\limits_{1}^{x}f(t)dt,x\in(1,\infin).\) Let α denote the number of solutions of the equation g(x) = 0 in the interval (1, 8] and \(β=\lim\limits_{x→1+}\frac{g(x)}{x-1}\). Then the value of α + β is equal to _____.- JEE Advanced - 2024
- Integral Calculus
- A dimensionless quantity is constructed in terms of electronic charge \(e\), permittivity of free space \(\epsilon_0\) , Planck’s constant ℎ, and speed of light c. If the dimensionless quantity is written as \(e^\alpha\epsilon_0^\beta h^\gamma c^\delta\)and n is a non-zero integer, then\((\alpha, \beta,\gamma,\delta)\) is given by
- JEE Advanced - 2024
- Semiconductor electronics: materials, devices and simple circuits
- A block of mass \(5 kg\) moves along the \(x-\)direction subject to the force \(F = (−20x + 10) N,\) with the value of \(x \) in metre. At time \(t = 0 s,\) it is at rest at position \(x = 1 m\). The position and momentum of the block at \(t = (\pi/4)\) s are
- JEE Advanced - 2024
- Work-energy theorem
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.

Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
Concepts Used:
Introduction to Organic Chemistry
Organic chemistry is the branch of chemistry that involves the scientific study of organic compounds. Organic chemistry primarily deals with the structure and chemical composition of organic compounds, the physical and chemical properties of organic compounds, and the chemical reactions undergone by these compounds.
Reaction Intermediates in Organic Chemistry:
Intermediates can be understood as the first product of a consecutive reaction. For example, in a chemical reaction, if A→B and B→C, then, B can be said to be the intermediate for reaction A→C. The reactions in organic chemistry occur via the formation of these intermediates.
Reagents in Organic Chemistry:
Reagents are the chemicals that we add to bring about a specific change to an organic molecule. Any general reaction in organic chemistry can be written as:
Substrate + Reagent → Product
Where the substrate is an organic molecule to which we add the reagent. Based on the ability to either donate or abstract electrons, the reagents can be classified as:
- Electrophiles
- Nucleophiles






