The correct option(s) about entropy (S) is(are)
$[ R =$ gas constant, $F =$ Faraday constant, $T =$ Temperature $]$
$[ R =$ gas constant, $F =$ Faraday constant, $T =$ Temperature $]$
- For the reaction, $M (s)+2 H ^{+}(a q) \rightarrow H _2(g)+ M ^{2+}(a q)$, if $\frac{ dE _{\text {cell }}}{ dT }=\frac{ R }{ F }$, then the entropy change of the reaction is $R$ (assume that entropy and internal energy changes are temperature independent).
- The cell reaction, $Pt (s)\left| H _2(g, 1 bar )\right| H ^{+}(a q, 0.01 M ) \| H ^{+}(a q, 0.1 M )\left| H _2(g, 1 bar )\right| Pt (s)$, is an entropy driven process.
- For racemization of an optically active compound, $\Delta S >0$.
- $\Delta S >0$, for $\left[ Ni \left( H _2 O \right)_6\right]^{2+}+3 en \rightarrow\left[ Ni ( en )_3\right]^{2+}+6 H _2 O$ (where en $=$ ethylenediamine).
The Correct Option is B, C, D
Solution and Explanation
The correct options are
(B) The cell reaction, $Pt (s)\left| H _2(g, 1 bar )\right| H ^{+}(a q, 0.01 M ) \| H ^{+}(a q, 0.1 M )\left| H _2(g, 1 bar )\right| Pt (s)$, is an entropy driven process.
(C) For racemization of an optically active compound, $\Delta S >0$.
(D) $\Delta S >0$, for $\left[ Ni \left( H _2 O \right)_6\right]^{2+}+3 en \rightarrow\left[ Ni ( en )_3\right]^{2+}+6 H _2 O$ (where en $=$ ethylenediamine).
Top Questions on Electrochemistry
- What is the numerical value of one Faraday in Coulombs?
- CUET (UG) - 2024
- Chemistry
- Electrochemistry
- Read the following passage and answer the next five questions based on it.
Battery or cell converts chemical energy of the redox reaction to electrical energy. In fuel cells (a galvanic cell), the chemical energy of combustion of fuels like H2, ethanol, etc., is directly converted to electrical energy. In a fuel cell, H2 and O2 react to produce electricity, where H2 gas is oxidized at the anode and oxygen is reduced at the cathode, and the reactions involved are:
Anode reaction: H2 + 2OH- → 2H2O + 2e-
Cathode reaction: O2 + 2H2O + 4e- → 4OH-
67.2 L of H2 at STP reacts in 15 minutes.
- CUET (UG) - 2024
- Chemistry
- Electrochemistry
- In the cell reaction
P+(𝑎𝑞)+Q(𝑠)→P(𝑠)+Q+(𝑎𝑞)
the EMF of the cell, 𝐸𝑐𝑒𝑙𝑙 is zero. The standard EMF of the cell, 𝐸𝑜𝑐𝑒𝑙𝑙 is
[Given:
Activities of all solids are unity.
Activity of P+(𝑎𝑞) is 2 M. Activity of Q+(𝑎𝑞) is 1 M.
𝑅 = universal gas constant; 𝑇 = temperature; 𝐹 = Faraday constant]- IIT JAM CY - 2024
- Physical Chemistry
- Electrochemistry
- A salt QCl of a certain metal Q is electrolyzed to its elements. 40 g of metal Q is formed at an electrode. The volume of Cl2 formed at the other electrode at 1 atm pressure and 298 K is _______ litres. (rounded off to one decimal place)
[Given: The gas constant 𝑅 = 0.082 L atm mol−1 K−1 , the molar mass of Q is 40 g mol−1 and Cl2 is assumed to be an ideal gas]- IIT JAM CY - 2024
- Physical Chemistry
- Electrochemistry
- Mobility of ions Li+ , Na+ , K+ , Ag+ in water at 298 K follows the order
- IIT JAM CY - 2024
- Physical Chemistry
- Electrochemistry
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
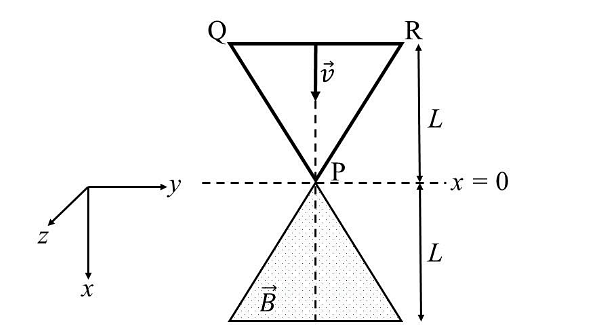
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability



