Question:
ΔS° (in J mol-1 K-1) for the given reaction at 298 K is ________.
[Cu(H2O)6]2+ + en ⇌ [Cu(H2O)4(en)]2+ + 2H2O
(Given: log K1 = 10.6, where K1 is the equilibrium constant. ΔH° =-54 kJ mol-1 and R = 8.314 J mol-1 K-1)
(rounded off to two decimal places)
ΔS° (in J mol-1 K-1) for the given reaction at 298 K is ________.
[Cu(H2O)6]2+ + en ⇌ [Cu(H2O)4(en)]2+ + 2H2O
(Given: log K1 = 10.6, where K1 is the equilibrium constant. ΔH° =-54 kJ mol-1 and R = 8.314 J mol-1 K-1)
(rounded off to two decimal places)
[Cu(H2O)6]2+ + en ⇌ [Cu(H2O)4(en)]2+ + 2H2O
(Given: log K1 = 10.6, where K1 is the equilibrium constant. ΔH° =-54 kJ mol-1 and R = 8.314 J mol-1 K-1)
(rounded off to two decimal places)
Updated On: Jul 12, 2024
Hide Solution
Verified By Collegedunia
Correct Answer: 21.4
Solution and Explanation
The correct answer is 21.40 to 22.00 (approx).
Was this answer helpful?
0
0
Top Questions on Thermodynamic functions and their relationships
- In thermogravimetric analysis, 12.45 mg of CuSO4.5H2O was subjected to heating under N2 atmosphere. At a particular temperature, there was a weight loss of 3.6 mg. The number of water molecule(s) lost per formula unit is _____.
(Given molar mass (in g mol-1) of H = 1.0, O = 16.0, S = 32.0, and Cu = 63.5)
(rounded off to the nearest integer)- GATE CY - 2024
- Physical Chemistry
- Thermodynamic functions and their relationships
- 2 mol of a monoatomic ideal gas with initial volume of 5 L and pressure 10 bar undergoes an irreversible adiabatic expansion against a constant final pressure of 1 bar. The final volume (in L) is _______.
(Given: R = 8.314 × 10-2 L bar mol-1 K-1)
(rounded off to one decimal place)- GATE CY - 2024
- Physical Chemistry
- Thermodynamic functions and their relationships
- The correct statement(s) related to an ensemble is (are) :
- GATE CY - 2024
- Physical Chemistry
- Thermodynamic functions and their relationships

k1=2k2, find the initial concentration of A.- GATE CY
- Physical Chemistry
- Thermodynamic functions and their relationships
Questions Asked in GATE CY exam
- The order and the number of classes present in a group with the irreducible representations A1, A2, B1, B2, E1, and E2, are, respectively,
- GATE CY - 2024
- Mass spectrometry
- In the EPR spectrum of an aqueous solution of VOSO4 at room temperature, the total number of hyperfine splitting signals is
- GATE CY - 2024
- Nuclear magnetic resonance: Principles
- The partial vapor pressure of 0.1 molal solution of B in liquid A is 60 kPa at 300 K. The partial vapor pressure (in kPa) of a solution containing B with mole fraction of 0.1 in liquid A at 300 K is ______.
(Assume the solute B obeys Henry's law. The molar mass of A is 80 g mol-1.)
(rounded off to three decimal places)- GATE CY - 2024
- Ideal and Non-ideal solutions
- 1H NMR spectrum of a mixture containing CH3Br (x mol) and (CH3)3CBr (y mol) shows two singlets at 2.7 ppm and 1.8 ppm, with the relative ratio of 3:1 (integration value), respectively. The value of x/y is ______.
(rounded off to the nearest integer)- GATE CY - 2024
- NMR and ESR spectroscopy
- The reaction(s) that yield(s) X as the major product is (are)
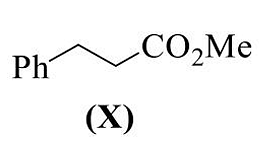
- GATE CY - 2024
- Identification of products, intermediates and isotopic labelling
View More Questions



