Question:
polyethylene-glycol is used in the preparation of which types of detergent?
polyethylene-glycol is used in the preparation of which types of detergent?
Updated On: Apr 30, 2024
- Anionic detergent
- Non-ionic detergent
- Cationic detergent
- Soap
Hide Solution
Verified By Collegedunia
The Correct Option is B
Solution and Explanation
The correct answer is option (B): Non-ionic detergent
Nonionic detergents are detergents that do not carry any electric charge in the molecular structure. They have both hydrophobic and hydrophilic regions in the molecule. Examples of nonionic detergents are Tween 20, and Triton X-100. Polyethylene glycol is a water-soluble polymer and has repeating units of ethylene glycols. It is a non-ionic substance and is used for the preparation of non–ionic detergents, due to the presence of the -OH group.
Nonionic detergents are detergents that do not carry any electric charge in the molecular structure. They have both hydrophobic and hydrophilic regions in the molecule. Examples of nonionic detergents are Tween 20, and Triton X-100. Polyethylene glycol is a water-soluble polymer and has repeating units of ethylene glycols. It is a non-ionic substance and is used for the preparation of non–ionic detergents, due to the presence of the -OH group.
Was this answer helpful?
0
0
Top Questions on Chemistry in Everyday Life
- Match List I with List IIChoose the correct answer from the options given below:
LIST I (Name of polymer) LIST II (Uses) A Glyptal I Flexible pipes B Neoprene II Synthetic wool C Acrilan III Paints and Lacquers D LDP IV Gaskets - JEE Main - 2023
- Chemistry
- Chemistry in Everyday Life
- $Nd ^{2+}=$
- JEE Main - 2023
- Chemistry
- Chemistry in Everyday Life
- Which of the following compounds are not used as disinfectants?
A. Chloroxylenol
B. Bithional
C. Veronal
D. Prontosil
E. Terpineol
Choose the correct answer from the options given below:- JEE Main - 2023
- Chemistry
- Chemistry in Everyday Life
- Given below are two statements :
Statement I : Upon heating a borax bead dipped in cupric sulphate in a luminous flame, the colour of the bead becomes green.
Statement II : The green colour observerd is due to the formation of copper(I) metaborate.
In the light of the above statements, choose the most appropriate answer from the options given below :- JEE Main - 2023
- Chemistry
- Chemistry in Everyday Life
Benzyl isocyanide can be obtained by : Choose the correct answer from the options given below :
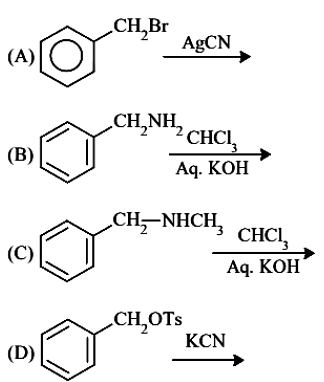
- JEE Main - 2023
- Chemistry
- Chemistry in Everyday Life
View More Questions
Questions Asked in GUJCET exam
- What is the de Broglie wavelength associated with an electron accelerated through a potential difference of 64 volts?
- GUJCET - 2023
- Refraction of Light
- Which of the following ore is not in oxide form?
- GUJCET - 2023
- General Principles and Processes of Isolation of Elements
- Which one is a reaction to prepare CCl2F2(freon-12) from CCl4?
- GUJCET - 2023
- Haloalkanes and Haloarenes
- Methylamine reacts with HNO2 to form?
- Which of the following are peroxo acids of sulpher?
- GUJCET - 2023
- p -Block Elements
View More Questions
Concepts Used:
Chemistry in Everyday Life
The scientific study of matter’s properties and behaviour is known as chemistry. It is a natural science that studies the elements that makeup matter, as well as the compounds, made up of atoms, molecules, and ions: their composition, structure, qualities, and behaviour, as well as the changes that occur when they mix with other things.
- Importance of Chemistry in Food - Chemicals are the fundamental components of everything. Chemical molecules make up all food, including carbs, vitamins, lipids, proteins, and fibre, which are all safe and often desirable.
- Importance of Chemistry in Medicines - Medicines or pharmaceuticals are chemical substances that are used to treat diseases and relieve pain. Chemistry has made significant contributions to health care. Chemistry, for example, aids in the manufacture and application of surgical materials.
- Importance of Chemistry in Cosmetics - In our daily lives, we use lotions, fragrances, talcum powder, and a variety of other cosmetic goods. All of these items are developed in laboratories using chemicals for our health and skin.
- Importance of Chemistry in Soaps and Detergents - Soaps are sodium and potassium salts of fatty acids with greater molecular weights, such as stearic acid, palmitic acid, and oleic acid. Sodium salts of long-chain alkyl hydrogen sulphates or sodium salts of long-chain alkyl benzene sulphonic acids are commonly used as detergents.



