Question:
In a bimolecular reaction, the steric factor $P$ was experimentally determined to be $4.5.$ The correct option(s) among the following is(are)
In a bimolecular reaction, the steric factor $P$ was experimentally determined to be $4.5.$ The correct option(s) among the following is(are)
Updated On: Jun 14, 2022
- The activation energy of the reaction is unaffected by the value of the steric factor
- Experimentally determined value of frequency factor is higher than that predicted by Arrhenius equation
- Since P = 4.5, the reaction will not proceed unless an effective catalyst is used
- The value of frequency factor predicted by Arrhenius equation is higher than that determined experimentally
Hide Solution
Verified By Collegedunia
The Correct Option is B
Solution and Explanation
[A] The activation energy of the reaction is unaffected by the value of the steric factor
[B] Experimentally determined value of frequency factor is higher than that predicted by Arrhenius equation
[B] Experimentally determined value of frequency factor is higher than that predicted by Arrhenius equation
Was this answer helpful?
0
0
Top Questions on kinetics equations
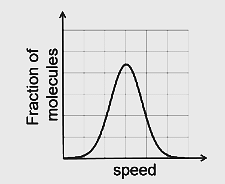
- If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is
- JEE Advanced - 2020
- Chemistry
- kinetics equations
- The time required to complete 3/4th of first order reaction is 32 min. then find \(t_{\frac{1}{2}}\)
- JIPMER MBBS - 2018
- Chemistry
- kinetics equations
- The half life period of a first order chemical reaction is 6.93 minutes. The time required for the completion of 99% of the chemical reaction will be (log 2 = 0.301)
- VITEEE - 2017
- Chemistry
- kinetics equations
- The integrated rate equation is $ kt=\log \,C_{0}-\log C_{t} $ . The straight line graph is obtained by plotting
- Haryana PMT - 2008
- Chemistry
- kinetics equations
- The coordinates of a moving particle at any time t are given by $ x=\alpha {{t}^{3}} $ and $ y={{t}^{3}}. $ The speed of the particle at time t is given by
- Rajasthan PMT - 2008
- Chemistry
- kinetics equations
View More Questions
Questions Asked in JEE Advanced exam
- Let \(S=\left\{\begin{pmatrix} 0 & 1 & c \\ 1 & a & d\\ 1 & b & e \end{pmatrix}:a,b,c,d,e\in\left\{0,1\right\}\ \text{and} |A|\in \left\{-1,1\right\}\right\}\), where |A| denotes the determinant of A. Then the number of elements in S is _______.
- JEE Advanced - 2024
- Matrices
- A block of mass \(5 kg\) moves along the \(x-\)direction subject to the force \(F = (−20x + 10) N,\) with the value of \(x \) in metre. At time \(t = 0 s,\) it is at rest at position \(x = 1 m\). The position and momentum of the block at \(t = (\pi/4)\) s are
- JEE Advanced - 2024
- Work-energy theorem
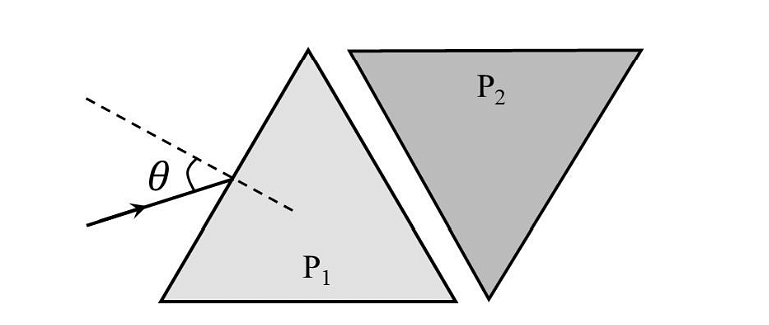
- Two equilateral-triangular prisms \(P_1 \)and \(P_2\) are kept with their sides parallel to each other, in vacuum, as shown in the figure. A light ray enters prism \(P_1\) at an angle of incidence 𝜃 such that the outgoing ray undergoes minimum deviation in prism \(P_2\). If the respective refractive indices of \(P_1\) and\( P_2\) are \(√ 3 /2\) and \(√3\), then \(\theta = sin{−1}[\sqrt \frac{ 3}{ 2} sin ( \frac{\pi}{B} )],\) where the value of \(\beta\) is ______.
- JEE Advanced - 2024
- Ray optics and optical instruments
- Let \(\overrightarrow{OP}=\frac{\alpha-1}{\alpha}\hat{i}+\hat{j}+\hat{k},\overrightarrow{OQ}=\hat{i}+\frac{\beta-1}{\beta}\hat{j}+\hat{k}\) and \(\overrightarrow{OR}=\hat{i}+\hat{j}+\frac{1}{2}\hat{k}\) be three vector where α, β ∈ R - {0} and 0 denotes the origin. If \((\overrightarrow{OP}\times\overrightarrow{OQ}).\overrightarrow{OR}=0\) and the point (α, β, 2) lies on the plane 3x + 3y - z + l = 0, then the value of l is _______.
- JEE Advanced - 2024
- Vector Algebra
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
View More Questions
Concepts Used:
Kinetics Equations
It is branch of physics that defines motion with respect to space and time is known as kinematics.
Inverse Kinematics: Inverse Kinematics do the reverse of kinematics.
There are four basic kinematics equations:
Rotational Kinematics Equations
Another branch of kinematics equations which deals with the rotational motion of anybody.