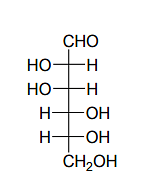
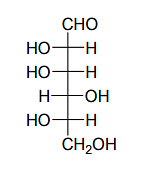
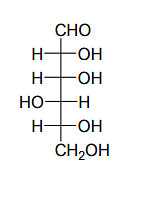
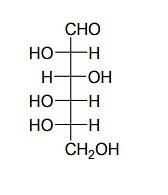Given:
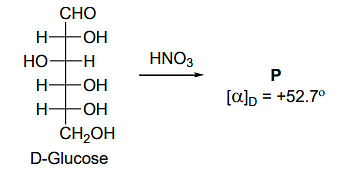
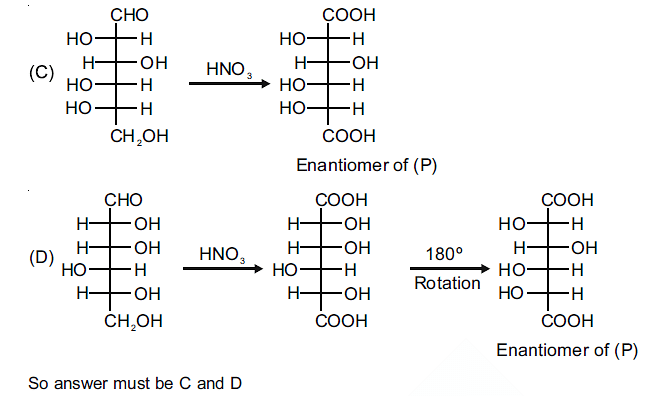
The compound(s), which on reaction with HNO3 will give the product having a degree of rotation, [α]D = –52.7º is(are);
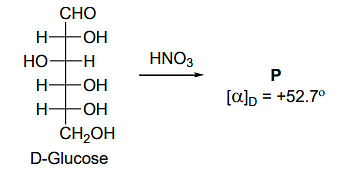
The compound(s), which on reaction with HNO3 will give the product having a degree of rotation, [α]D = –52.7º is(are);
The Correct Option is C, D
Solution and Explanation
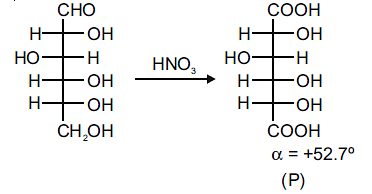 |
|
The enantiomer of (P) will have –52.7º rotation. So the reactant must be an isomer of D-glucose which can given the mirror image of (P)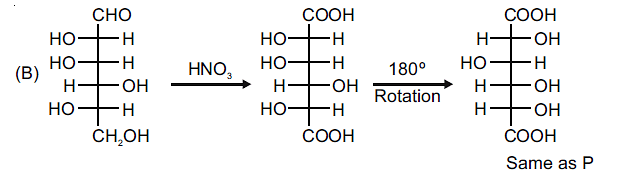
Top Questions on Glucose
- Which one of the following products is NOT obtained in anaerobic decomposition of glucose ?
- The ∆G´ and $K´_{eq}$ values of ATP hydrolysis are -32.34 kJ $mol^{-1}$ and $4.6 x10^5$, respectively. The ∆G´ and $K´_{eq}$ values of enzymatic hydrolysis of glucose-6- phosphate to glucose and phosphate are $-13.18 kJ mol^{-1}$ and 203.8, respectively. The ∆G´ value of reaction of glucose-6-phosphate formation from glucose and ATP by hexokinase is ____ $kJ mol^{-1}$ (rounded off to 2 decimal places). [All reactions are carried out at pH 7.0 and 25 °C].
- The correct representation in six-membered pyranose form for the following sugar $ [X]$ is

- Treatment of D-glucose with aqueous NaOH results in a mixture of monosaccharides, which are
- Which one of the following statements is TRUE with regard to glucose?
Questions Asked in JEE Advanced exam
- Let \(S=\left\{\begin{pmatrix} 0 & 1 & c \\ 1 & a & d\\ 1 & b & e \end{pmatrix}:a,b,c,d,e\in\left\{0,1\right\}\ \text{and} |A|\in \left\{-1,1\right\}\right\}\), where |A| denotes the determinant of A. Then the number of elements in S is _______.
- JEE Advanced - 2024
- Matrices
- A block of mass \(5 kg\) moves along the \(x-\)direction subject to the force \(F = (−20x + 10) N,\) with the value of \(x \) in metre. At time \(t = 0 s,\) it is at rest at position \(x = 1 m\). The position and momentum of the block at \(t = (\pi/4)\) s are
- JEE Advanced - 2024
- Work-energy theorem
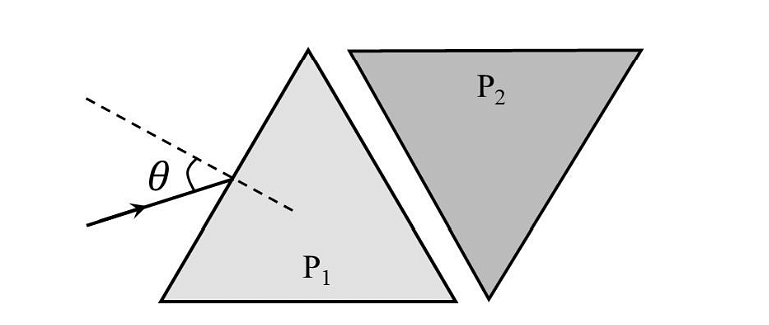
- Two equilateral-triangular prisms \(P_1 \)and \(P_2\) are kept with their sides parallel to each other, in vacuum, as shown in the figure. A light ray enters prism \(P_1\) at an angle of incidence 𝜃 such that the outgoing ray undergoes minimum deviation in prism \(P_2\). If the respective refractive indices of \(P_1\) and\( P_2\) are \(√ 3 /2\) and \(√3\), then \(\theta = sin{−1}[\sqrt \frac{ 3}{ 2} sin ( \frac{\pi}{B} )],\) where the value of \(\beta\) is ______.
- JEE Advanced - 2024
- Ray optics and optical instruments
- Let \(\overrightarrow{OP}=\frac{\alpha-1}{\alpha}\hat{i}+\hat{j}+\hat{k},\overrightarrow{OQ}=\hat{i}+\frac{\beta-1}{\beta}\hat{j}+\hat{k}\) and \(\overrightarrow{OR}=\hat{i}+\hat{j}+\frac{1}{2}\hat{k}\) be three vector where α, β ∈ R - {0} and 0 denotes the origin. If \((\overrightarrow{OP}\times\overrightarrow{OQ}).\overrightarrow{OR}=0\) and the point (α, β, 2) lies on the plane 3x + 3y - z + l = 0, then the value of l is _______.
- JEE Advanced - 2024
- Vector Algebra
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
Concepts Used:
Glucose
Glucose is a simple sugar, also known as dextrose, that is a primary source of energy for living organisms. It is a monosaccharide, meaning it consists of a single sugar unit, and is chemically classified as an aldohexose, which means it has six carbon atoms and an aldehyde functional group.
Glucose is produced by plants through the process of photosynthesis, where it is synthesized from carbon dioxide and water using energy from sunlight. It is also produced in the human body through the breakdown of complex carbohydrates, such as starch and glycogen, in the process of digestion.
Glucose is transported throughout the body via the bloodstream and taken up by cells where it is metabolized to produce energy in the form of ATP. Excess glucose can be stored in the liver and muscles as glycogen for later use.
Read Also: Structure of Glucose and Fructose
Glucose is an important component of many foods, such as fruits, honey, and sweetened beverages. It is also used as a sweetener in the food industry and as a medical treatment for hypoglycemia, a condition characterized by low blood glucose levels.
Measurement of glucose levels in the blood is an important diagnostic tool for monitoring and managing diabetes, a chronic metabolic disorder characterized by high blood glucose levels. Glucose testing can be done using a variety of methods, such as fingerstick testing and continuous glucose monitoring.