For the reaction:
$I ^{-}+ ClO _{3}^{-}+ H _{2} SO _{4} \longrightarrow Cl ^{-}+ HSO _{4}^{-}+ I _{2}$
The correct statement(s) in the balanced equation is/are:
- stoichiometric coefficient of $HSO_4^-$ is $6$
- iodide is oxidised
- sulphur is reduced
- $H_2O$ is one of the products
The Correct Option is D
Solution and Explanation
$ClO _{3}^{-}+6 I ^{-}+6 H _{2} SO _{4} \longrightarrow 3 I _{2}+ Cl ^{-}+6 HSO _{4}^{-}+3 H _{2} O$
Top Questions on Redox reactions
- Consider the following redox reaction:\[\text{MnO}_4^- + \text{H}^+ + \text{H}_2 \text{C}_2 \text{O}_4 \rightleftharpoons \text{Mn}^{2+} + \text{H}_2 \text{O} + \text{CO}_2\]The standard reduction potentials are given as below (E\(_\text{red}\)):\[E^\circ_{\text{MnO}_4^-/\text{Mn}^{2+}} = +1.51 \, \text{V}\]\[E^\circ_{\text{CO}_2/\text{H}_2 \text{C}_2 \text{O}_4} = -0.49 \, \text{V}\]If the equilibrium constant of the above reaction is given as \(K_\text{eq} = 10^x\), then the value of (x = ________ ) (nearest integer).
- JEE Main - 2024
- Chemistry
- Redox reactions
- Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): In aqueous solutions \( \text{Cr}^{2+} \) is reducing while \( \text{Mn}^{3+} \) is oxidising in nature.
Reason (R): Extra stability to half-filled electronic configuration is observed than incompletely filled electronic configuration.
In the light of the above statement, choose the most appropriate answer from the options given below:- JEE Main - 2024
- Chemistry
- Redox reactions
- The number of ions from the following that have the ability to liberate hydrogen from a dilute acid is _______. Ti2+, Cr2+ and V2+.
- JEE Main - 2024
- Chemistry
- Redox reactions
- Only \( 2 \, \text{mL} \) of \( \text{KMnO}_4 \) solution of unknown molarity is required to reach the end point of a titration of \( 20 \, \text{mL} \) of oxalic acid (\( 2 \, \text{M} \)) in acidic medium. The molarity of \( \text{KMnO}_4 \) solution should be ______ \( \text{M} \).
- JEE Main - 2024
- Chemistry
- Redox reactions
- When \( \text{MnO}_2 \) and \( \text{H}_2\text{SO}_4 \) is added to a salt (A), the greenish yellow gas liberated as salt (A) is:
- JEE Main - 2024
- Chemistry
- Redox reactions
Questions Asked in JEE Advanced exam
- A closed vessel contains 10 g of an ideal gas X at 300 K, which exerts 2 atm pressure. At the same temperature, 80 g of another ideal gas Y is added to it and the pressure becomes 6 atm. The ratio of root mean square velocities of X and Y at 300 K is
- JEE Advanced - 2024
- States of matter
- Let the function \(f:[1,\infin)→\R\) be defined by
\(f(t) = \begin{cases} (-1)^{n+1}2, & \text{if } t=2n-1,n\in\N, \\ \frac{(2n+1-t)}{2}f(2n-1)+\frac{(t-(2n-1))}{2}f(2n+1) & \text{if } 2n-1<t<2n+1,n\in\N. \end{cases}\)
Define \(g(x)=\int\limits_{1}^{x}f(t)dt,x\in(1,\infin).\) Let α denote the number of solutions of the equation g(x) = 0 in the interval (1, 8] and \(β=\lim\limits_{x→1+}\frac{g(x)}{x-1}\). Then the value of α + β is equal to _____.- JEE Advanced - 2024
- Integral Calculus
- A dimensionless quantity is constructed in terms of electronic charge \(e\), permittivity of free space \(\epsilon_0\) , Planck’s constant ℎ, and speed of light c. If the dimensionless quantity is written as \(e^\alpha\epsilon_0^\beta h^\gamma c^\delta\)and n is a non-zero integer, then\((\alpha, \beta,\gamma,\delta)\) is given by
- JEE Advanced - 2024
- Semiconductor electronics: materials, devices and simple circuits
- A block of mass \(5 kg\) moves along the \(x-\)direction subject to the force \(F = (−20x + 10) N,\) with the value of \(x \) in metre. At time \(t = 0 s,\) it is at rest at position \(x = 1 m\). The position and momentum of the block at \(t = (\pi/4)\) s are
- JEE Advanced - 2024
- Work-energy theorem
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
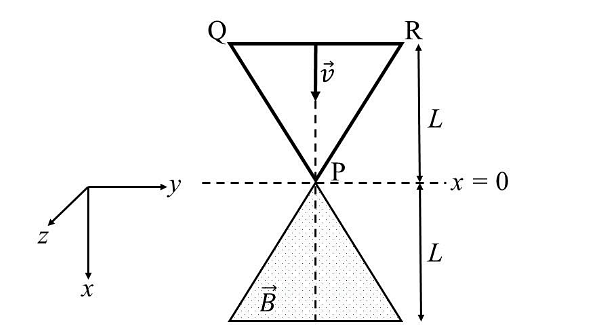
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0?
Concepts Used:
Redox Reactions
Redox Reaction:
Redox reactions are chemical reactions where oxidation and reduction take place simultaneously. In this type of reaction, there is a gain of electrons for one chemical species while the other loses electrons or simply involves transfer of electrons. The species that loses electrons is oxidized while the one that gains electrons is reduced.
Types of Redox Reactions:
Redox reactions can be differentiated into 4 categories namely combination reactions, decomposition reactions, displacement reactions, and disproportionation reactions. Each is explained separately below:
Combination Reaction:
In this, the molecules combine to form new compounds. For example, when magnesium reacts to nitrogen.
Decomposition Reaction:
Opposite to the combination reaction, here there is a breakdown of compounds to simpler substances. For example, electrolysis of water.
Displacement Reaction:
In this, the more reactive metal will displace the less reactive one in a chemical reaction. The reactivity of an element is represented in a series called the reactivity series (arranged in decreasing order of reactivity) which makes it easier to determine the chemical reaction and its products.
Disproportionation Reaction:
This is a peculiar type of reaction where an element showing a particular oxidation state will be oxidized and reduced simultaneously. Another thing to note is that these reactions will always have an element that can exhibit three oxidation states.



