For the gas phase reaction, $ C_2 H_4 + H_2 \rightleftharpoons C_2 H_6 $ $ \hspace15mm$ $ ( \triangle H = - 32.7 \, kcal )$ carried out in a vessel, the equilibrium concentration of $ C_2H_4$ can be increased by
- increasing the temperature
- decreasing the pressure
- removing some $ H_2$
- adding some $C_2 H_6$
The Correct Option is D
Solution and Explanation
The above reaction is exothermic, increasing temperature will favour backward reaction, will increase the amount of $C_2H_4$ .
Decreasing pressure will favour reaction in direction containing more molecules (reactant side in the present case). Therefore, decreasing pressure will increase amount of $ C_2 H_4$ .
Removing $H_2$, which is a reactant, will favour reaction in backward direction, more $C_2 H_4$ will be formed.
Adding $C_2 H_6$ will favour backward reaction and some of the $ C_2 H_6 $ will be dehydrogenated to $ C_2 H_4$ .
Top Questions on Equilibrium
- The effect of addition of helium gas to the following reaction in equilibrium state, is : $PCl _5( g ) \rightleftharpoons PCl _3( g )+ Cl _2( g )$
- JEE Main - 2023
- Chemistry
- Equilibrium
- Consider the following equation: $2 SO _2(g)+ O _2(g) \rightleftharpoons 2 SO _3(g), \Delta H =-190 \,kJ$. The number of factors which will increase the yield of $SO _3$ at equilibrium from the following is _______
A. Increasing temperature
B. Increasing pressure
C. Adding more $SO _2$
D. Adding more $O _2$ E Addition of catalyst- JEE Main - 2023
- Chemistry
- Equilibrium
- The dissociation constant of acetic acid is $x \times 10^{-5}$ When $25 \,mL$ of $0.2 \,M \,CH _3 COONa$ solution is mixed with $25 \,mL$ of $0.02\, M \,CH _3 COOH$ solution, the $pH$ of the resultant solution is found to be equal to 5. The value of $x$ is ___
- JEE Main - 2023
- Chemistry
- Equilibrium
- $20 \,mL$ of $01 \,M \,NH _4 OH$ is mixed with $40\, mL$ of $005\, M \,HCl$ The $pH$ of the mixture is nearest to: (Given: $K _{ b }\left( NH _4 OH \right)=1 \times 10^{-5}, \log 2=030$, $\log 3=048, \log 5=069, \log 7=084$, $\log 11=104$ )
- JEE Main - 2023
- Chemistry
- Equilibrium
- \(K_{sp}\) of \(BaSO_4\) is 8 × \(10^{-11}\). If the solubility in presence of 0.1 M \(CaSO_4\) is?
- JEE Main - 2023
- Chemistry
- Equilibrium
Questions Asked in JEE Advanced exam
- A region in the form of an equilateral triangle (in x-y plane) of height L has a uniform magnetic field 𝐵⃗ pointing in the +z-direction. A conducting loop PQR, in the form of an equilateral triangle of the same height 𝐿, is placed in the x-y plane with its vertex P at x = 0 in the orientation shown in the figure. At 𝑡 = 0, the loop starts entering the region of the magnetic field with a uniform velocity 𝑣 along the +x-direction. The plane of the loop and its orientation remain unchanged throughout its motion.
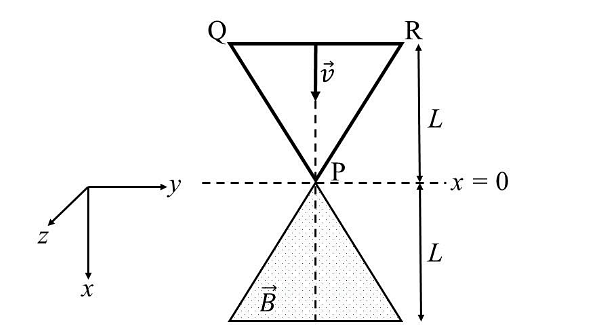
Which of the following graph best depicts the variation of the induced emf (E) in the loop as a function of the distance (𝑥) starting from 𝑥 = 0? - Two beads, each with charge q and mass m, are on a horizontal, frictionless, non-conducting, circular hoop of radius R. One of the beads is glued to the hoop at some point, while the other one performs small oscillations about its equilibrium position along the hoop. The square of the angular frequency of the small oscillations is given by [ \(\epsilon_0 \)is the permittivity of free space.]
- JEE Advanced - 2024
- Moving charges and magnetism
- A group of 9 students, s1, s2,…., s9, is to be divided to form three teams X, Y and Z of sizes 2, 3, and 4, respectively. Suppose that s1 cannot be selected for the team X and s2 cannot be selected for the team Y. Then the number of ways to form such teams, is _______.
- JEE Advanced - 2024
- Combinations
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
- Let X be a random variable, and let P(X = x) denote the probability that X takes the value x. Suppose that the points (x, P(X = x)), x = 0,1,2,3,4, lie on a fixed straight line in the xy -plane, and P(X = x) = 0 for all x ∈ R - {0,1,2,3,4}. If the mean of X is \(\frac{5}{2}\) , and the variance of X is α, then the value of 24α is ______.
- JEE Advanced - 2024
- Probability
Concepts Used:
Equilibrium
An equilibrium represents a state in a process when the observable properties such as color, temperature, pressure, concentration etc do not show any change.
The word equilibrium means ‘balance’ which indicates that a chemical reaction represents a balance between the reactants and products taking part in the reaction. The equilibrium state is also noticed in certain physical processes such as the melting point of ice at 0℃, both ice and water are present at equilibrium.
In the case of physical processes such as the melting of solid, dissolution of salt in water etc., the equilibrium is called physical equilibrium while the equilibrium associated with chemical reaction is known as chemical equilibrium.
Equilibrium in Chemical changes
The chemical equilibrium in a reversible reaction is the state at which both forward and backward reactions occur at the same speed.
The stage of the reversible reaction at which the concentration of the reactants and products do not change with time is called the equilibrium state.
Read More: Calculating Equilibrium Concentration
Types of Chemical Equilibrium
There are two types of chemical equilibrium:
- Homogeneous Equilibrium
- Heterogeneous Equilibrium
Homogenous Chemical Equilibrium
In this type, the reactants and the products of chemical equilibrium are all in the same phase. Homogenous equilibrium can be further divided into two types: Reactions in which the number of molecules of the products is equal to the number of molecules of the reactants. For example,
- H2 (g) + I2 (g) ⇌ 2HI (g)
- N2 (g) + O2 (g) ⇌ 2NO (g)
Reactions in which the number of molecules of the products is not equal to the total number of reactant molecules. For example,
- 2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
- COCl2 (g) ⇌ CO (g) + Cl2 (g)
Heterogeneous Chemical Equilibrium
In this type, the reactants and the products of chemical equilibrium are present in different phases. A few examples of heterogeneous equilibrium are listed below.
- CO2 (g) + C (s) ⇌ 2CO (g)
- CaCO3 (s) ⇌ CaO (s) + CO2 (g)
Thus, the different types of chemical equilibrium are based on the phase of the reactants and products.
Check Out: Equilibrium Important Questions



