A hammer of mass $200\, kg$ strikes a steel block of mass $200\, g$ with a velocity $8\, ms^{-1}$. If $23\%$ of the energy is utilized to heat the steel block, the rise in temperature of the block is (specific heat capacity of steel = $460 \; J \; kg^{-1} \; K^{-1}$)
- 8 K
- 16 K
- 12 K
- 24 K
The Correct Option is B
Solution and Explanation
steel block of the mass $=200 \,g =0.2 \,kg$
and specific heat capacity of steel, $s=460 \,J \,kg ^{-1} K ^{-1}$
Velocity of hammer, $v=8 \,ms ^{-1}$
As we know that, Kinetic energy, $KE =\frac{1}{2} m v^{2}$
Putting the given values, we get
$=\frac{1}{2} \times 200 \times 8^{2}=6400\, J$
Hence, the $23 \%$ of this energy is converted to heat.
$\Rightarrow H=\frac{6400 \times 23}{100}=1472\, J$
The rise in temperature of steel,
$\Delta T=\frac{H}{m s}=\frac{1472}{460 \times 0.2}=16\, K$
Hence, the rise in temperature is $16\, K$.
Top Questions on specific heat capacity
- Two moles a monoatomic gas is mixed with six moles of a diatomic gas. The molar specific heat of the mixture at constant volume is :
- JEE Main - 2024
- Physics
- specific heat capacity
- The circuit shown in the figure contains an inductor L, a capacitor \(C_0\), a resistor\( R_0\) and an ideal battery. The circuit also contains two keys \(K_1\) and \(K_2\). Initially, both the keys are open and there is no charge on the capacitor. At an instant, key \(K_1\) is closed and immediately after this the current in \(R_0\) is found to be \(I_1\). After a long time, the current attains a steady state value \(I_2\). Thereafter,\( K_2\) is closed and simultaneously \(K_1\) is opened and the voltage across \(C_0\) oscillates with amplitude \(C_0\) and angular frequency \(\omega_0\).
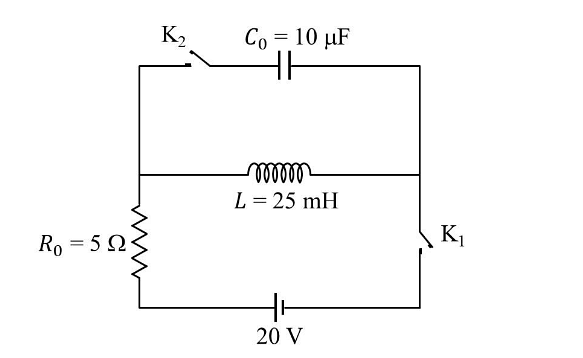
Match the quantities mentioned in List-I with their values in List-II and choose the correct option.List-I List-II P The value of \(I1\) in Ampere is I \(0\) Q The value of I2 in Ampere is II \(2\) R The value of \(\omega_0\) in kilo-radians/s is III \(4\) S The value of \(V_0\) in Volt is IV \(20\) 200 - JEE Advanced - 2024
- Physics
- specific heat capacity
- Let $\gamma_1$ be the ratio of molar specific heat at constant pressure and molar specific heat at constant volume of a monoatomic gas and $\gamma_2$ be the similar ratio of diatomic gas Considering the diatomic gas molecule as a rigid rotator, the ratio, $\frac{\gamma_1}{\gamma_2}$ is :
- JEE Main - 2023
- Physics
- specific heat capacity
The pressure of a gas changes linearly with volume from $A$ to $B$ as shown in figure If no heat is supplied to or extracted from the gas then change in the internal energy of the gas will be Is
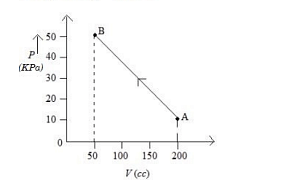
- JEE Main - 2023
- Physics
- specific heat capacity
- A water heater of power $2000 W$ is used to heat water. The specific heat capacity of water is $4200 J$ $kg ^{-1} K ^{-1}$ .The efficiency of heater is $70 \%$ .Time required to heat $2 kg$ of water from $10^{\circ} C$ to $60^{\circ} C$ is___$ s$
(Assume that the specific heat capacity of water remains constant over the temperature range of the water)- JEE Main - 2023
- Physics
- specific heat capacity
Questions Asked in DUET exam
- What is the shape and magnetic nature of permanganate ion ?
- DUET - 2019
- d -and f -Block Elements
- The mobility of charge carriers increases with
- DUET - 2011
- Electric Current
- Phagocytosis and pinocytosis are collectively termed as
- DUET - 2011
- Cell: the unit of life
- How many $ 1\,\mu F $ capacitors must be connected in parallel to store a charge of $1\, C$ with a potential of $110\, V$ across the capacitors?
- DUET - 2011
- electrostatic potential and capacitance
- In the given structure of a compound, the correct various bond moments direction involving are shown as
- DUET - 2011
- Chemical bonding and molecular structure
Concepts Used:
Specific Heat Capacity
Specific heat of a solid or liquid is the amount of heat that raises the temperature of a unit mass of the solid through 1°C.
Molar Specific Heat:
The Molar specific heat of a solid or liquid of a material is the heat that you provide to raise the temperature of one mole of solid or liquid through 1K or 1°C.
Specific Heat at Constant Pressure or Volume:
The volume of solid remains constant when heated through a small range of temperature. This is known as specific heat at a constant volume. It is denoted as CV.
The pressure of solid remains constant when heated through a small range of temperature. This is known as specific heat at constant pressure which can be denoted as CP.



