Which of the following statement(s) is(are) correct about the spectrum of hydrogen atom?
The ratio of the longest wavelength to the shortest wavelength in Balmer series is $\frac{9}5$
- There is an overlap between the wavelength ranges of Balmer and Paschen series
- The wavelength of Lyman series are given by $\left(1+\frac{1}{ m ^{2}}\right) \lambda_{0}$, where $\lambda_{0}$ is the shortest wavelength of Lyman series and $m$ is an integer
- The wavelength ranges of Lyman and Balmer series do not overlap
The Correct Option is D
Solution and Explanation
The correct answer is option (D): The wavelength ranges of Lyman and Balmer series do not overlap
Top Questions on Dual nature of radiation and matter
The work functions of Caesium (Cs), Potassium (K) and Sodium (Na) are 2.14eV, 2.30 eV and 2.75eV respectively. If incident electromagnetic radiation has an incident energy of 2.20eV, which of these photosensitive surfaces may emit photoelectrons?
- NEET (UG) - 2023
- Physics
- Dual nature of radiation and matter
- When two monochromatic light of frequency ν and \(\frac{ν}{2}\) are incident on a photoelectric metal, their stopping potential becomes \(\frac{V_s}{2}\) and Vs respectively. The threshold frequency for this metal is:
- NEET (UG) - 2023
- Physics
- Dual nature of radiation and matter
- A metal exposed to light of wavelength $800\, nm$ and emits photoelectrons with a certain kinetic energy The maximum kinetic energy of photo-electron doubles when light of wavelength $500\, nm$ is used The work function of the metal is (Take $hc =1230 \,eV - nm )$
- JEE Main - 2023
- Physics
- Dual nature of radiation and matter
- From the photoelectric effect experiment, following observations are made Identify which of these are correct
A. The stopping potential depends only on the work function of the metal
B. The saturation current increases as the intensity of incident light increases
C. The maximum kinetic energy of a photo electron depends on the intensity of the incident light
D. Photoelectric effect can be explained using wave theory of light
Choose the correct answer from the options given below:- JEE Main - 2023
- Physics
- Dual nature of radiation and matter
A laser source emits light of wavelength 300nm and has a power of 3.3mW. The average number of photons emitted per second is:(Speed of light-3x108m/s,Plank's constant 6.6 x 10-34J/s)
- KEAM - 2023
- Physics
- Dual nature of radiation and matter
Questions Asked in JEE Advanced exam
- Let \(S=\left\{\begin{pmatrix} 0 & 1 & c \\ 1 & a & d\\ 1 & b & e \end{pmatrix}:a,b,c,d,e\in\left\{0,1\right\}\ \text{and} |A|\in \left\{-1,1\right\}\right\}\), where |A| denotes the determinant of A. Then the number of elements in S is _______.
- JEE Advanced - 2024
- Matrices
- A block of mass \(5 kg\) moves along the \(x-\)direction subject to the force \(F = (−20x + 10) N,\) with the value of \(x \) in metre. At time \(t = 0 s,\) it is at rest at position \(x = 1 m\). The position and momentum of the block at \(t = (\pi/4)\) s are
- JEE Advanced - 2024
- Work-energy theorem
- Two equilateral-triangular prisms \(P_1 \)and \(P_2\) are kept with their sides parallel to each other, in vacuum, as shown in the figure. A light ray enters prism \(P_1\) at an angle of incidence 𝜃 such that the outgoing ray undergoes minimum deviation in prism \(P_2\). If the respective refractive indices of \(P_1\) and\( P_2\) are \(√ 3 /2\) and \(√3\), then \(\theta = sin{−1}[\sqrt \frac{ 3}{ 2} sin ( \frac{\pi}{B} )],\) where the value of \(\beta\) is ______.
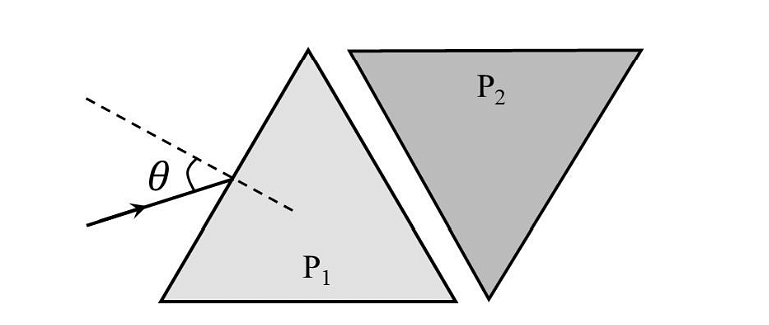
- JEE Advanced - 2024
- Ray optics and optical instruments
- Let \(\overrightarrow{OP}=\frac{\alpha-1}{\alpha}\hat{i}+\hat{j}+\hat{k},\overrightarrow{OQ}=\hat{i}+\frac{\beta-1}{\beta}\hat{j}+\hat{k}\) and \(\overrightarrow{OR}=\hat{i}+\hat{j}+\frac{1}{2}\hat{k}\) be three vector where α, β ∈ R - {0} and 0 denotes the origin. If \((\overrightarrow{OP}\times\overrightarrow{OQ}).\overrightarrow{OR}=0\) and the point (α, β, 2) lies on the plane 3x + 3y - z + l = 0, then the value of l is _______.
- JEE Advanced - 2024
- Vector Algebra
- Let \(\vec{p}=2\hat{i}+\hat{j}+3\hat{k}\) and \(\vec{q}=\hat{i}-\hat{j}+\hat{k}\). If for some real numbers α, β and γ we have
\(15\hat{i}+10\hat{j}+6\hat{k}=α(2\vec{p}+\vec{q})+β(\vec{p}-2\vec{q})+γ(\vec{p}\times\vec{q})\),
then the value of γ is ________.- JEE Advanced - 2024
- Vector Algebra
Concepts Used:
Dual Nature of Radiation and Matter
The dual nature of matter and the dual nature of radiation were throughgoing concepts of physics. At the beginning of the 20th century, scientists untangled one of the best-kept secrets of nature – the wave-particle duplexity or the dual nature of matter and radiation.
Electronic Emission
The least energy that is needed to emit an electron from the surface of a metal can be supplied to the loose electrons.
Photoelectric Effect
The photoelectric effect is a phenomenon that involves electrons getting away from the surface of materials.
Heisenberg’s Uncertainty Principle
Heisenberg’s Uncertainty Principle states that both the momentum and position of a particle cannot be determined simultaneously.



