An electron is revolving in its Bohr orbit having Bohr radius of 0.529 Å, then the radius of third orbit is
An electron is revolving in its Bohr orbit having Bohr radius of 0.529 Å, then the radius of third orbit is
- 4.761 Å
- 4234 nm
- 5125 nm
- 4496 Å
The Correct Option is A
Solution and Explanation
To determine the radius of the third orbit in the Bohr model of an atom, we can use the following formula:
rn = r1 * n2
where:
rn is the radius of the nth orbit
r1 is the Bohr radius (0.529 Å)
n is the principal quantum number (orbit number)
For the third orbit (n = 3), the formula becomes:
r3 = r1 x 32 = 0.529 Å x 9
Calculating the value, we get:
r3 = 4.761 Å
Therefore, the radius of the third orbit is 4.761 Å.
So, the correct option is (A) 4.761 Å.
Top Questions on atom structure models
- Crystal structures of two metals A and B are two-dimensional square lattices with same lattice constant a. Electrons in metals behave as free electrons. The Fermi surfaces corresponding to A and B are shown by solid circles in figures.

The electron concentrations in A and B are \( n_A \) and \( n_B \), respectively. The value of \((n_B / n_A)\) is:- GATE PH - 2024
- Atomic and Molecular Physics
- atom structure models
- The mean distance between the two atoms of an HD molecule is \( r \), where H and D denote hydrogen and deuterium, respectively. The mass of the hydrogen atom is \( m_H \). The energy difference between the two lowest lying rotational states of HD in multiples of \(\frac{h^2}{m_H r^2}\) is:
- GATE PH - 2024
- Atomic and Molecular Physics
- atom structure models
An amplitude-modulated wave is represented by cm (t) = 10[1+0.6 sin(1250t)] sin(103 t). Then the modulation index is:
- TS EAMCET - 2023
- Physics
- atom structure models
- A hydrogen-like atom of atomic number Z transits from n=4 to n=3. The photon emitted from this transition is incident on a metal surface of threshold wavelength λth = 310nm. If the maximum kinetic energy of the emitted electron is 1.95ev, find the value of Z.
- JEE Advanced - 2023
- Physics
- atom structure models
- A hydrogen-like atom of atomic number Z transits from n=4 to n=3. The photon emitted from this transition is incident on a metal surface of threshold wavelength λth = 310nm. If the maximum kinetic energy of the emitted electron is 1.95ev, find the value of Z.
- JEE Advanced - 2023
- Physics
- atom structure models
Questions Asked in KCET exam
- The current in a coil changes from 2A to 5A in 0.3s. The magnitude of emf induced in the coil is 1.0V. The value of self-inductance of the coil is
- KCET - 2023
- Electromagnetic induction
- A stretched wire of a material whose Young's modulus Y = 2 × 1011 Nm-2 has Poisson's ratio of 0.25. Its lateral strain εl = 10-3. The elastic energy density of the wire is
- KCET - 2023
- mechanical properties of solids
- A particle moves along the curve \(\frac{x^2}{16}+\frac{y^2}{4}=1\). When the rate of change of abscissa is 4 times that of its ordinate, then the quadrant in which the particle lies is
- KCET - 2023
- Conic sections
- A point object is moving at a constant speed of 1 ms-1 along the principal axis of a convex lens of focal length 10cm. The speed of the image is also 1 ms-1 , when the object is at _______ cm from the optic centre of the lens.
- KCET - 2023
- spherical lenses
- A cubical Gaussian surface has side of length a = 10 cm. Electric field lines are parallel to x-axis as shown. The magnitudes of electric fields through surfaces ABCD and EFGH are 6kNC-1 and 9kNC-1 respectively. Then the total charge enclosed by the cube is
[Take ε0 = 9 × 10-12 Fm-1]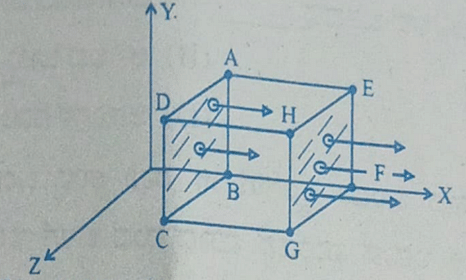
- KCET - 2023
- Gauss Law
Concepts Used:
Atom Structure Models
The three atomic models are as follows:
Thomson model:
Thomson atomic model was proposed by William Thomson in the year 1900. This model explained the description of an inner structure of the atom theoretically. It was strongly supported by Sir Joseph Thomson, who had discovered the electron earlier.
Thomson assumed that an electron is two thousand times lighter than a proton and believed that an atom is made up of thousands of electrons. In this atomic structure model, he considered atoms surrounded by a cloud having positive as well as negative charges. The demonstration of the ionization of air by X-ray was also done by him together with Rutherford. They were the first to demonstrate it. Thomson’s model of an atom is similar to a plum pudding.
Rutherford’s Alpha Scattering Experiment:
Rutherford’s conducted an experiment by bombarding a thin sheet of gold with α-particles and then studied the trajectory of these particles after their interaction with the gold foil.
Bohr’s Model of an Atom:
Bohr model of the atom was proposed by Neil Bohr in 1915. It came into existence with the modification of Rutherford’s model of an atom. Rutherford’s model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons.



