
Content Strategy Manager
Protein is found in muscles, bones, skin, hair, and almost every other organ or tissue in the body. It is a component of enzymes that promote many chemical reactions, similar to hemoglobin, which carries oxygen in the blood. Amino acids refer to more than 20 basic components that makeup proteins. One can't store amino acids, so our system makes them in two ways: from scratch or by modifying other amino acids. Essential amino acids such as histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine must be taken from the diet.
Key Terms: Protein, amino acids, structure, condensation, myosin, muscle, polypeptide, receptor, immune, mutation.
Structure Of Protein
[Click Here for Sample Questions]
Peptide bonds are formed by the condensation of amino acids into protein structures. A peptide bond (CONH) is formed between the amine group of one molecule and the carboxyl group of an adjacent molecule, removing the water molecule.
Otherwise, it is an amide bond. Polypeptide chains are formed when peptide bonds are formed between more than 10 amino acids. When the mass of a polypeptide chain exceeds 10000 units and the number of amino acids in the chain exceeds 100, a protein is formed.

Protein Structure
Read more:
| Other Concepts Related to Proteins | ||
|---|---|---|
| Difference Between Glucose and Fructose | Complex Carbohydrates | Zwitterion |
| Difference Between Fats and Oils | Nucleic Acids | Sucrose |
Classification Of Protein Structure
[Click Here for Previous Year Questions]
Proteins can be classified into two types according to their molecular shape.
1. Fibrous protein:
A fibrous structure is formed when the polypeptide chains are parallel and held together by hydrogen and disulfide bonds. These are water-insoluble proteins.
Examples – Keratin (contained in hair, wool, silk) and myosin (contained in muscle).

Fibrous Protein
2. Globular protein:
The structure of globular protein is formed when the chain of a polypeptide is curled into a sphere. These are usually water-soluble.
Example- Insulin and albumin are common examples of globular proteins.

Globular Protein
Level Of Protein Structure
[Click Here for Sample Questions]
The level of protein structure are mentioned below-
Primary structure of the protein
The primary structure of a protein is the exact arrangement of the amino acids that make up the chain. The exact order of the proteins is very important as it determines the final folding and therefore the function of the proteins.
- The number of polypeptide chains together form a protein. These chains have amino acids arranged in specific sequences that are characteristic of specific proteins. Changing the sequence changes the entire protein.
- The primary structure of a protein is the amino acid sequence of its polypeptide chain. If the protein was a piece of popcorn destined to decorate the Christmas tree, the main structure of the protein is a sequence of popcorn of different shapes and types spliced together.
- The covalent peptide bond that connects the amino acids maintains the primary structure of the protein.
- All documented hereditary disorders such as cystic fibrosis, sickle cell anemia, and leucoderma are caused by mutations that lead to changes in primary protein structure, then secondary, tertiary, and possibly quaternary. It leads to the change of the following structure.
- The amino acid is a small organic molecule composed of chiral carbon with four substituents. Of these, only the fourth side chain of the amino acid is different.
Secondary structure of the protein
Protein secondary structure refers to the locally folded structure formed within a polypeptide as a result of interactions between skeletal atoms. The protein is not only present in the simple strands of a polypeptide.
- These polypeptide chains are usually folded due to the interaction between the amine and carboxyl groups of the peptide bond. Structure refers to the form in which a long-chain polypeptide can exist.
- They are provided in two different types of structures: α-helical structure and β-folded sheet structure.
- This structure occurs due to the regular folding of the backbone of the polypeptide chain by hydrogen bonds between the CO and NH groups of the peptide bond.
- However, the segment of the protein chain can adopt its own local folding. It is much simpler and usually takes the form of a spiral, elongated shape, or loop.
- These local folds are called secondary elements and form the secondary structure of the protein.
(a) α–helix:
α–helix is the most common way in which the polypeptide chain twists into the right helix to form all possible hydrogen bonds and the NH group of each amino acid residue is formed. It is one. Hydrogen bonds to CO in adjacent turns of the helix. The polypeptide chain is twisted into the screw on the right.
(b) β-sheet:
In this arrangement, polypeptide chains are stretched side by side and connected by intermolecular hydrogen bonds. In this structure, all peptide chains are stretched to almost the maximum extent, then juxtaposed and bound by intermolecular hydrogen bonds. The structure is similar to curtain pleats, so it is called β-pleated fabric.
Tertiary structure of the protein
This structure is the result of further folding of the secondary structure of the protein.
- H-bonds, electrostatic forces, disulfide bonds, and van der Waals forces stabilize this structure.
- The tertiary structure of a protein represents the overall folding of the polypeptide chain and the further folding of the secondary structure.
- It gives rise to two primary molecular shapes called fibrous and spherical.
- The main forces that stabilize the secondary and tertiary structure of proteins are hydrogen bonds, disulfide bonds, van der Waals forces, and electrostatic attraction.
Quaternary structure of the protein
The quaternary structure results from the spatial arrangement of various tertiary structures. Some proteins are composed of two or more polypeptide chains called subunits.
- The spatial arrangement of these subunits with each other is called the quaternary structure.
- The exact amino acid sequence of each protein drives it to fold into a unique biologically active three-dimensional fold, also known as tertiary structure. Proteins are made up of various combinations of secondary elements, some simple and some more complex.
- The part of the protein chain that has its own 3D fold and can be assigned a function is called a "domain".
- These are now considered evolutionary and functional components of proteins.
- Many proteins are mostly enzymes and contain the organic or elemental components necessary for their activity and stability.
- Therefore, studying protein evolution not only provides structural insights but also links proteins from very different parts of metabolism.
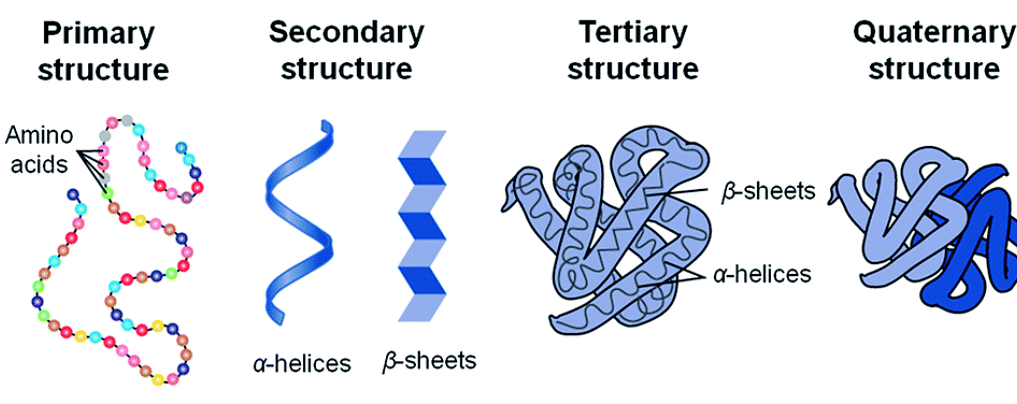
Level Of Protein Structure
Functions Of Protein
[Click Here for Previous Year Questions]
Here are some common functions of Protein-
- Protein is important for the execution of complex processes and the synthesis and regeneration of DNA. Enzymes are proteins that digest food. Protein is involved in the formation of many hormones that help control the body's constituents. Cells use surface receptors to communicate with each other and with the outside world.
- These receptors are made of proteins. Antibodies are proteins in the body that the immune system uses to repair and heal the body after the introduction of foreign pathogens. A protein that enables interactions between cells and organs.
Things to remember
- In particular, Linderstrom Lang (1952) first proposed a four-level hierarchy of protein structures: central, secondary, tertiary, and quaternary.
- The primary structure of the protein is the basic level of the hierarchy, which is a well-defined linear sequence of the amino acids that make up the polypeptide chain.
- Secondary structure is the next level of primary structure, which is the regular folding of a region into a particular structural pattern within a polypeptide chain. The hydrogen bond between the carbonyl oxygen and the amide hydrogen of the peptide bond is usually held together by a secondary structure.
- Tertiary structure is the next highest level of secondary structure, a specific three-dimensional arrangement of all amino acids within a single polypeptide chain. This structure is usually conformational, native, and active, and is grouped together by multiple non-covalent interactions.
- The quaternary structure is the next "level up" between two or more polypeptide chains from the tertiary structure, which is a specific spatial arrangement and interaction.
Previous Year's Questions
- The richest source of vitamin A are...[JIPMER 1998]
- Which of the following hormones is produced under the condition of stress which stimulates glycogenolysis in the liver of human beings?….[NEET 2014]
- Haemoglobin is….[NEET 1997]
- Which of the following can possibly be used as analgesic without causing addiction and mood modification?...[NEET 1997]
- Which functional group participates in disulphide bond formation in proteins?….[NEET 2005]
- The cell membranes are mainly composed of….[NEET 2005]
- Which of the following protein destroy the antigen when it enters in body cell?...[NEET 1995]
- The segment of DNA which acts as the instrumental manual for the synthesis of the protein is...[NEET 2009]
- Which one of the following is a peptide hormone...[NEET 2006]
- The human body does not produce...[NEET 2006]
- Enzymes take part in a reaction and….[NEET 1993]
- Which of the following is correct statement?...[NEET 2001]
- Which one of the following gives positive Fehling's solution test?….[NEET 2001]
- Enzymes are made up of….[NEET 2002]
- Which one of the following is an amine hormone?...[NEET 2008]
- Chargaff's rule states that in an organism...[NEET 2003]
- Glycolysis is...[NEET 2003]
- Phospholipids are esters of glycerol with...[NEET 2003]
- The correct statement in respect of protein haemoglobin is that it...[NEET 2004]
- The hormone that helps in the conversion of glucose to glycogen is...[NEET 2004]
Sample questions
Ques. What causes the denaturation of proteins? (4 marks)
Ans: The stability of a protein and its structure depends on physical and chemical conditions.
- Temperature and pH greatly affect their stability. Protein denaturation is a condition in which the unique three-dimensional structure of a protein changes. Changes in temperature, pH, or other chemical activity break the hydrogen bonds present in the protein. This leads to the expansion of globular proteins and the unwinding of the helical structure.
- The unwinding of helical structures affects the chemistry of proteins, which lose their biological activity. This phenomenon of losing activity due to physical or chemical changes and unraveling the helical structure is called protein denaturation. Denaturation of proteins destroys secondary and tertiary structures, preserving only primary structures. Covalent bonds are broken, preventing interactions between amino acid chains. This leads to a loss of the biological activity of the protein.
Ques. What is the protein structure made up of?
Name its different stages? (3 marks)
Ans: The structure of a protein is made up of the condensation of amino acids that form peptide bonds. The sequence of amino acids in a protein is called the primary structure.
Secondary structure is determined by the dihedral angle of the peptide bond, and tertiary structure is determined by the folding of the protein chain in space. The binding of folded polypeptide molecules to complex functional proteins results in a quaternary structure.
b.Four levels of protein structure. The major, secondary, tertiary, and quaternary levels of protein structure are four stages.
Ques. Is DNA a protein? How is a protein structure stabilized? (3 marks)
Ans: DNA is often linked to a protein in the cell nucleus called histones, but DNA itself is not a protein. Numbered DNA is a nucleic acid composed of phosphate groups, sugar groups, and bases (purines and pyrimidines), whereas proteins are large molecules composed of long chains of one or more amino acids.
Hydrogen bonds within the polypeptide chain and between the amino acid "R" groups help maintain the protein structure by maintaining the protein in the form formed by hydrophobic interactions. This type of bond creates so-called disulfide bridges.
Ques. Does temperature have any effect on the structure of the protein? What are the other factors that have an effect on the proteins? (4 marks)
Ans: The temperature has a significant effect on the structure of the protein. Changes in temperature denature proteins and alter their structure. The temperature however has no effect on the amino acid sequence in protein structure but it does affect the folding of the three-dimensional polypeptide chain. The non-polar hydrophobic interaction is broken by the temperature.
Heat in the presence and absence of carbohydrates, fluctuation in the pH ( especially alkaline), and exposure to oxidative conditions, including those generated by light and those caused by oxidizing lipids are the other environmental changes that harm the proteins.
Ques. Explain the primary and secondary structure of proteins? (4 marks)
Ans:
- Primary structure – The primary structure is defined as a sequence of amino acids that make up a polypeptide chain. Proteins are broken down into 20 amino acids. The order in which amino acids are present in a protein is called the primary sequence.
- Secondary structure – The regular repeating folding pattern of the backbone is called the secondary structure of the protein. The two most common folding patterns are the alpha helix and the beta-sheet.
- α-helix: A helix is the most common for forming all potential hydrogen bonds by twisting the polypeptide chain into the right helix and binding the NH group of each amino acid residue to the CO of the adjacent turn. It is one of the typical methods. Of the spiral. Upon rotation, the polypeptide chain formed the right spiral.
- Beta Sheets: The chains of the polypeptide are adjacent to each other and are thus connected by hydrogen bonds. All peptide chains are stretched to near maximum and placed side by side in this structure bound by Intermolecular hydrogen bonds. It is called β pleats because its structure resembles the pleats of a curtain.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check out:





Comments