The thermal capacity of any body is
- a measure of its capacity to absorb heat
- a measure of its capacity to provide heat
- the quantity of heat required to raise its temperature by a unit degree
- the quantity of heat required to raise the temperature of a unit mass of the body by a unit degree
The Correct Option is C
Solution and Explanation
Top Questions on specific heat capacity
- Two moles a monoatomic gas is mixed with six moles of a diatomic gas. The molar specific heat of the mixture at constant volume is :
- JEE Main - 2024
- Physics
- specific heat capacity
- The circuit shown in the figure contains an inductor L, a capacitor \(C_0\), a resistor\( R_0\) and an ideal battery. The circuit also contains two keys \(K_1\) and \(K_2\). Initially, both the keys are open and there is no charge on the capacitor. At an instant, key \(K_1\) is closed and immediately after this the current in \(R_0\) is found to be \(I_1\). After a long time, the current attains a steady state value \(I_2\). Thereafter,\( K_2\) is closed and simultaneously \(K_1\) is opened and the voltage across \(C_0\) oscillates with amplitude \(C_0\) and angular frequency \(\omega_0\).
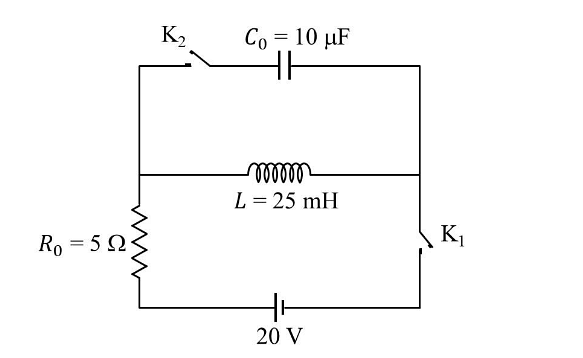
Match the quantities mentioned in List-I with their values in List-II and choose the correct option.List-I List-II P The value of \(I1\) in Ampere is I \(0\) Q The value of I2 in Ampere is II \(2\) R The value of \(\omega_0\) in kilo-radians/s is III \(4\) S The value of \(V_0\) in Volt is IV \(20\) 200 - JEE Advanced - 2024
- Physics
- specific heat capacity
- Let $\gamma_1$ be the ratio of molar specific heat at constant pressure and molar specific heat at constant volume of a monoatomic gas and $\gamma_2$ be the similar ratio of diatomic gas Considering the diatomic gas molecule as a rigid rotator, the ratio, $\frac{\gamma_1}{\gamma_2}$ is :
- JEE Main - 2023
- Physics
- specific heat capacity
The pressure of a gas changes linearly with volume from $A$ to $B$ as shown in figure If no heat is supplied to or extracted from the gas then change in the internal energy of the gas will be Is
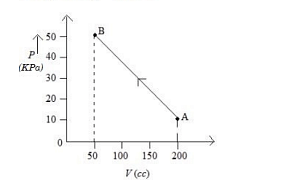
- JEE Main - 2023
- Physics
- specific heat capacity
- A water heater of power $2000 W$ is used to heat water. The specific heat capacity of water is $4200 J$ $kg ^{-1} K ^{-1}$ .The efficiency of heater is $70 \%$ .Time required to heat $2 kg$ of water from $10^{\circ} C$ to $60^{\circ} C$ is___$ s$
(Assume that the specific heat capacity of water remains constant over the temperature range of the water)- JEE Main - 2023
- Physics
- specific heat capacity
Questions Asked in Rajasthan PMT exam
- 300 cal of heat is supplied to raise the temperature of 50 g of air from $ 20{}^\circ C $ to $ 30{}^\circ C $ without any change in its volume. Change in internal energy per gram of air is
- Rajasthan PMT - 2011
- internal energy
- The velocity of water flowing in a non-uniform tube is 20 cm/s at a point where the tube radius is 0.2 cm. The velocity at another point, where the radius is 0.1 cm, is
- Rajasthan PMT - 2011
- mechanical properties of fluid
- Block A of mass 4 kg and block B of mass 6 kg are resting on a horizontal surface as shown in the figure. There is no friction between the block B and the horizontal surface. The coefficient of friction between the blocks is 0.2. If the value of $ g=10\,m/{{s}^{2}}, $ the maximum horizontal force F that can be applied on block B without any relative motion between A and B is
- The excess pressure inside a soap bubble A is twice that in another soap bubble B. The ratio of volumes of A and B is
- Rajasthan PMT - 2011
- Surface tension
- The unit of surface tension is
- Rajasthan PMT - 2011
- Surface tension
Concepts Used:
Specific Heat Capacity
Specific heat of a solid or liquid is the amount of heat that raises the temperature of a unit mass of the solid through 1°C.
Molar Specific Heat:
The Molar specific heat of a solid or liquid of a material is the heat that you provide to raise the temperature of one mole of solid or liquid through 1K or 1°C.
Specific Heat at Constant Pressure or Volume:
The volume of solid remains constant when heated through a small range of temperature. This is known as specific heat at a constant volume. It is denoted as CV.
The pressure of solid remains constant when heated through a small range of temperature. This is known as specific heat at constant pressure which can be denoted as CP.



