Electron belongs to the family of
Electron belongs to the family of
Mesons
Hyperons
Baryons
Leptons
The Correct Option is D
Solution and Explanation
The correct option is (D): Leptons
Top Questions on atom structure models
- The mean distance between the two atoms of an HD molecule is \( r \), where H and D denote hydrogen and deuterium, respectively. The mass of the hydrogen atom is \( m_H \). The energy difference between the two lowest lying rotational states of HD in multiples of \(\frac{h^2}{m_H r^2}\) is:
- GATE PH - 2024
- Atomic and Molecular Physics
- atom structure models
- Crystal structures of two metals A and B are two-dimensional square lattices with same lattice constant a. Electrons in metals behave as free electrons. The Fermi surfaces corresponding to A and B are shown by solid circles in figures.

The electron concentrations in A and B are \( n_A \) and \( n_B \), respectively. The value of \((n_B / n_A)\) is:- GATE PH - 2024
- Atomic and Molecular Physics
- atom structure models
- A hydrogen-like atom of atomic number Z transits from n=4 to n=3. The photon emitted from this transition is incident on a metal surface of threshold wavelength λth = 310nm. If the maximum kinetic energy of the emitted electron is 1.95ev, find the value of Z.
- JEE Advanced - 2023
- Physics
- atom structure models
An amplitude-modulated wave is represented by cm (t) = 10[1+0.6 sin(1250t)] sin(103 t). Then the modulation index is:
- TS EAMCET - 2023
- Physics
- atom structure models
- A hydrogen-like atom of atomic number Z transits from n=4 to n=3. The photon emitted from this transition is incident on a metal surface of threshold wavelength λth = 310nm. If the maximum kinetic energy of the emitted electron is 1.95ev, find the value of Z.
- JEE Advanced - 2023
- Physics
- atom structure models
Questions Asked in SRMJEEE exam
Which of the following statement is true for aqueous solution of 0.1 M urea, 0.2 M glucose nad 0.3 M sucrose
- SRMJEEE - 2023
- Biomolecules
The molar conductivities at infinite dilution for Na2SO4,K2S04,KCl, HCl and HCOONa at 300K are 260, 308, 150, 426, and 105 S cm2 mol-1, respectively. What will be A+m for formic acid in the same unit?
- SRMJEEE - 2023
- Electrochemistry
Electrophilic halogenation of phenol does not require catalyst because
- SRMJEEE - 2023
- Haloalkanes and Haloarenes
- Which one is the correct expression of electric potential on the axial point of a dipole whose dipole moment is р and length is 2l, at a distance of r?
- SRMJEEE - 2023
- Electrostatics
- If VA and VVB are the potentials of the two points A and B, what will be the correct relation between the electric field and the potential difference between the points? The distance between the points is d.
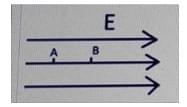
- SRMJEEE - 2023
- Electrostatics
Concepts Used:
Atom Structure Models
The three atomic models are as follows:
Thomson model:
Thomson atomic model was proposed by William Thomson in the year 1900. This model explained the description of an inner structure of the atom theoretically. It was strongly supported by Sir Joseph Thomson, who had discovered the electron earlier.
Thomson assumed that an electron is two thousand times lighter than a proton and believed that an atom is made up of thousands of electrons. In this atomic structure model, he considered atoms surrounded by a cloud having positive as well as negative charges. The demonstration of the ionization of air by X-ray was also done by him together with Rutherford. They were the first to demonstrate it. Thomson’s model of an atom is similar to a plum pudding.
Rutherford’s Alpha Scattering Experiment:
Rutherford’s conducted an experiment by bombarding a thin sheet of gold with α-particles and then studied the trajectory of these particles after their interaction with the gold foil.
Bohr’s Model of an Atom:
Bohr model of the atom was proposed by Neil Bohr in 1915. It came into existence with the modification of Rutherford’s model of an atom. Rutherford’s model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons.



