Alkyl halides react with dialkyl copper reagents to give
Show Hint
The reaction between alkyl halides and dialkyl copper reagents allows for the synthesis of alkanes, which are saturated hydrocarbons.
- alkenes
- alkyl copper halides
- alkanes
- alkenyl halides.
The Correct Option is C
Approach Solution - 1
The correct answer is Option C) alkanes
In Corey House's synthesis of alkane, alkyl halide reacts with lithium dialkyl cuprate. \(R_{2}CuLi+R' X \to RR'+RCu+LiX\)
For example, Lithium dialkyl cuprate reacts with ethyl chloride to give n-butane.
Li(CH3-CH2)2Cu + CH3-CH2-Cl →CH3-CH2-CH2-CH3 +LiCl + CH3-CH2-Cu
Discover More From Chapter: Haloalkanes and Haloarenes
Approach Solution -2
The correct answer is Option C) alkanes
Real Life Applications
The reaction of alkyl halides with dialkyl copper reagents to give alkanes is called Wittig reaction. It is used for the synthesis of alkanes.
1) It helps in the synthesis of steroids. Steroids are a class of organic compounds that are essential for many biological processes, including the production of sex hormones and the regulation of metabolism. The Wittig reaction can be used to synthesize a wide variety of steroids, including testosterone, estrogen, and cortisone.
2) Polymers are large molecules that are made up of repeating units. The Wittig reaction can be used to synthesize polymers that are used in a variety of applications, including plastics, rubbers, and adhesives. 
The Wittig reaction is also used in the synthesis of other organic compounds, such as pharmaceuticals and agrochemicals. It is a versatile reaction that can be used to synthesize a wide variety of compounds with different properties.
Question can also be asked as
- What are the products of the reaction between alkyl halides and dialkyl copper reagents?
- What is the Corey-House synthesis?
- How are alkanes synthesized from alkyl halides?
Approach Solution -3
The correct answer is Option C) alkanes
The reaction between alkyl halides and dialkyl copper reagents allows for the synthesis of alkanes, which are saturated hydrocarbons.
- Alkyl halides are organic compounds that contain a halogen atom (chlorine, bromine, or iodine) bonded to a carbon atom.
- They are widely used as starting materials in various organic reactions due to the reactivity of the halogen atom.
Dialkyl Copper Reagents
- Dialkyl copper reagents are organometallic compounds containing a copper atom bonded to alkyl groups.
- Common examples include diethyl copper (CuEt2) and dimethyl copper (CuMe2).
Alkyl Halides with Dialkyl Copper Reagents
When alkyl halides are treated with dialkyl copper reagents, a substitution reaction occurs.
The copper reagent acts as a nucleophile, attacking the alkyl halide and replacing the halogen atom with an alkyl group from the copper reagent.
Formation of Alkanes
- The key product of the reaction between alkyl halides and dialkyl copper reagents is an alkane.
- Alkanes are saturated hydrocarbons, meaning they consist solely of carbon and hydrogen atoms with only single bonds.
Importance in Organic Synthesis
- The synthesis of alkanes through the reaction of alkyl halides with dialkyl copper reagents provides a useful method for constructing carbon-carbon bonds.
- This reaction allows chemists to introduce alkyl groups into organic molecules, enabling the synthesis of complex organic compounds.
- The reaction is most effective with primary alkyl halides, as they are more reactive towards nucleophilic substitution.
- Secondary and tertiary alkyl halides may undergo competing elimination reactions.
Read More:
Learn with videos:
Top Questions on Haloalkanes and Haloarenes
- For SN2 reaction, the increasing order of the reactivity of the following alkyl halides is:
(A) CH3CH2CH2CH2Br (B) CH3CH2CH(Br)CH3 (C) (CH3)3CBr (D) (CH3)2CHCH2Br- CUET (UG) - 2024
- Chemistry
- Haloalkanes and Haloarenes
- Match List-I with List-II:

- CUET (UG) - 2024
- Chemistry
- Haloalkanes and Haloarenes
- Although chlorine is electron-withdrawing, it is ortho- and para-directing in electrophilic substitution because • (A) Chlorine withdraws electrons through inductive effect.
(B) Chlorine destabilizes the intermediate carbocation formed during electrophilic sub-stitution.
(C) Chlorine accepts electrons through resonance
(D) Chlorine releases electrons through resonance.
Choose the correct answer from the options given below:- CUET (UG) - 2024
- Chemistry
- Haloalkanes and Haloarenes
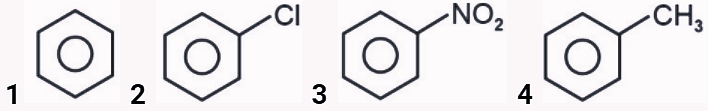
- What will be the reactivity order of following compounds towards electrophilic substitution reaction ?
- JEE Main - 2024
- Chemistry
- Haloalkanes and Haloarenes
- Which of the following sets of elements can be detected by Lassaigne's Test ?
- JEE Main - 2024
- Chemistry
- Haloalkanes and Haloarenes
Questions Asked in AIEEE exam
- A steel wire can sustain $100\, kg$ weight without breaking. If the wire is cut into two equal parts, each part can sustain a weight of
- AIEEE - 2012
- mechanical properties of solids
- The limiting line in Balmer series will have a frequency of (Rydberg constant, $R_{\infty}=3.29\times10^{15}$ cycles/s)
- AIEEE - 2012
- Electromagnetic Spectrum
- Which of the plots shown in the figure represents speed (v) of the electron in a hydrogen atom as a function of the principal quantum number (n)?
- The ratio of number of oxygen atoms (O) in 16.0 g ozone $(O_3), \,28.0\, g$ carbon monoxide $(CO)$ and $16.0$ oxygen $(O_2)$ is (Atomic mass: $C = 12,0 = 16$ and Avogadro?? constant $N_A = 6.0 x 10^{23}\, mol^{-1}$)
- AIEEE - 2012
- Mole concept and Molar Masses
- The compressibility factor for a real gas at high pressure is :
- AIEEE - 2012
- Van Der Waals equation
Concepts Used:
Haloalkanes and Haloarenes
The hydrocarbons such as Haloalkanes and Haloarenes are the ones, in which one or more hydrogen atoms are replaced with halogen atoms. The main difference between Haloalkanes and Haloarenes is that Haloalkanes are derived from open chained hydrocarbons, also called alkanes, and Haloarenes are derived from aromatic hydrocarbons.
- Haloalkanes have hydrocarbons made up of aliphatic alkanes and one or more hydrogen atoms replaced by halogens (elements such as Chlorine, Bromine, Fluorine, Iodine, etc.) whereas, haloarenes consist of aromatic ring or rings and one or more hydrogen atoms replaced by halogens.
- In haloalkanes, the halogen atom is attached to the sp3 hybridized carbon atom of the alkyl group whereas, in haloarenes, the halogen atom is attached to the sp3 hybridized carbon atom of the alkyl group.
- Haloalkanes are saturated organic compounds where all the chemical bonds are attached to the carbon atom with single bonds and a single carbon atom is attached to the Halogen atom, whereas, the haloarenes differ from Haloalkanes by their method of preparation and properties.
- Haloalkanes are made by aliphatic alkanes by the process of free radical halogenation, whereas, haloarenes are made by direct halogenation of aromatic rings.
- Haloalkanes are odorless compounds, whereas, haloarenes have a sweet odor.
- Haloalkanes precipitate in SN2 substitution reactions, whereas, haloarenes do not precipitate in SN2 substitution reactions.
- Example of haloalkanes is CH3Cl (Methyl Chloride) and CH3CH2Br (Ethyl Bromide) and the example of haloarenes is Chlorobenzene, Bromobenzene.