
Content Curator
Percentage Yield Formula shows what proportion of the product is obtained compared to the utmost possible mass. The atom economy of a reaction gives the percentage of atoms in reactants that form the desired product. Percent yield refers to the percent ratio of the weight of the product obtained to the theoretical yield.
Read More: Number of Moles Formula
| Table of Content |
Key Terms: Percentage Yield, Theoretical Yield, Actual Yield, Molar Mass, Mole Ratio, Percentage Atom Economy, Limiting Reactant
Percentage Yield Formula
[Click Here for Sample Questions]
Percent yield formula in a chemical reaction can be calculated by dividing the actual yield by the theoretical yield, and then by multiplying it by 100. Thus, Percent Yield is the percentage ratio of actual yield to the theoretical yield. The formula for calculating percentage yield is:
| (Actual Yield / Theoretical Yield) x 100% |
If the actual and theoretical yield is the same, the percent yield is 100%. Generally, the percent yield is lower than 100% because the actual yield is usually less than the theoretical value.

Percentage Yield Formula ExampleExample: Define Percent Yield by the given Decomposition Reaction: MgCO3 → MgO + CO2 Solution: The above reaction shows that for 1 mole of reactant (MgCO3), we can get 1 mole of product, that is MgO. Simply, the reactant and the product can be expressed as 1:1 mole ratio. With the amount of reactant mentioned, we can determine the Theoretical Yield in comparison with the mole ratio. Thus, the ratio of the Actual Yield with the Theoretical Yield gives Percentage Yield. |
Read More:
Theoretical Yield Formula
[Click Here for Sample Questions]
Theoretical yield formula is the maximum possible mass of a product that can be made in a chemical reaction. It can start with a balanced chemical equation by defining the limiting reactant in the process. Theoretical yield formula can be given by:
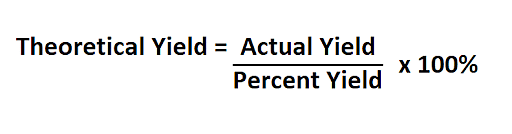
Given below is the step-by-step method of calculating theoretical yield:
- Use the molar mass of the reactant to convert grams of reactant into moles of reactant.
- Using the mole ratio between reactant and product, convert moles of reactant to moles of product.
- Using the molar mass of the product, convert moles of product to grams of the product.
The theoretical yield can be calculated using the equation:
Wt. of product (in grams) = Wt. of reactant in grams (1 mol reactant / molar mass of reactant) x (mole ratio of product/reactant) x (molar mass of product / 1 mol product)
Also check:
| Relation between Molarity and Molality | Mole fraction | Relation between Normality and Molarity |
| VSEPR Theory | Ionic Bond (Electrovalent Bond) | Combined Gas Law |
Percentage Atom Economy Formula
[Click Here for Sample Questions]
Atom economy refers to the percentage of the entire mass of reactants that is successfully converted to the specified product. This can be calculated by taking the ratio of the mass of the utilized atoms to the total mass of the atoms of all the reactants and multiplying by 100.
The percentage atom economy formula is given as:
| Atom Economy = (Mass of atom in desired Product / Mass of atom in Reactants) x 100% |
The major difference between atom economy and percentage yield is that the atom economy is calculated by dividing the molar mass of the desired product by the molar mass of all the reactants, whereas percentage yield is calculated by diving the actual yield of the product from the theoretical yield of the product.
Read More:
Things to Remember
- Percent yields are less than 100%. However, percent yields greater than 100% are possible if the measured product of the reaction contains impurities that cause its mass to be greater than it actually would be if the product would be pure.
- A limiting reagent is a chemical reactant that limits the quantity of product that is formed. The limiting reagent gives the smallest yield of product calculated from the reagents (reactants) available. This smallest yield of product is called Theoretical Yield.
- Theoretical yield is calculated based on the stoichiometry of the chemical equation, whereas actual yield is experimentally determined.
- Usually, the actual yield is less than the theoretical yield because not many reactions proceed to completion, that is, are not 100% efficient.
Also Check:
Sample Questions
Ques: During a chemical reaction, 0.5 g of product is made. The maximum calculated yield is 1.6 g. What is the percent yield of this reaction? (2 marks)
Ans. We know that according to Percent Yield Formula,
Percentage yield = (Actual yield/Theoretical yield)× 100%
= 0.5/1.6× 100%
= 31.25%
Therefore, the percentage yield of this reaction is 31.25%.
Ques: During a chemical reaction 1.8 g of product is made. The maximum calculated yield is 3.6 g. What is the percent yield of this reaction? (2 marks)
Ans. We know that according to Percent Yield Formula,
Percentage yield = (Actual yield/Theoretical yield)× 100%
= 1.8/3.6× 100%
= 50%
Therefore, the percentage yield of this reaction is 50%.
Ques: If the percentage yield is 45% with the theoretical yield as 4g, what would the actual yield be? Calculate using the percentage yield formula. (2 marks)
Ans. Using the percentage yield formula,
Percentage yield = (Actual yield/Theoretical yield) × 100%
45 = Actual yield / 4 × 100
Actual yield = 1.8
Therefore, the actual yield is 1.8g
Ques: During a chemical reaction, 0.9 g of product is made. The maximum calculated yield is 1.8g. Calculate the percentage yield of this reaction by using the percent yield formula chemistry? (2 marks)
Ans. Substitute the values in the corresponding percentage yield formula,
Percentage yield = (Actual Yield / Theoretical Yield) x 100%
Percentage yield = (0.9 / 1.8) x 100%
Percentage yield = 0.5 x 100%
Percentage yield = 50%
Ques: Determine the theoretical yield of the formation of geranyl formate from 465 g of geraniol. A chemist making geranyl formate uses 465 g of starting material and collects 419g of purified product. Percentage yield is given as 92.1%. (3 marks)
Ans. The actual yield is 419 g which is the quantity of the desired product.
Percentage yield is 92.1%
Therefore, by using the theoretical yield formula chemistry is as,
Theoretical yield = (Actual Yield / Percentage Yield) x 100
Theoretical yield = (419 / 92.1) x 100
Theoretical yield = 4.545 x 100
Theoretical yield = 454 g
Ques: What is the percent yield of the following reaction if 60 grams of CaCO3 is heated to give 15 grams of CaO? (3 marks)
Ans: CaCO3→ CaO + CO2
Ideally, how many grams of CaO should be produced? First verify the equation is balanced; it is. Now convert to moles, based on the amount of CaCO3 present.
× = 0.6 mole CaO
= 33.6 grams CaO
So, ideally, 33.6 grams of CaO should have been produced in this reaction. This is the theoretical yield. However, the problem tells us that only 15 grams were produced. 15 grams is the actual yield. It is now a simple matter to find percent yield.
= 0.446 = 44.6%
Ques: Calculate the percent yield of sodium sulfate when 32.18 g of sulfuric acid reacts with excess sodium hydroxide to produce 37.91 g of sodium sulfate. (3 marks)
Ans. First, note that the question clearly states that sodium hydroxide is the excess reagent. You always can ignore a reactant if the problem says it’s in excess. That’s like a big “this-one-isn’t-important” sign in the problem.
So sulfuric acid is the limiting reagent and is the reagent you should use to calculate the theoretical yield:
Theory predicts that 46.59 g of sodium sulfate product is possible if the reaction proceeds perfectly and to completion. But the question states that the actual yield is only 37.91 g of sodium sulfate. With these two pieces of information, you can calculate the percent yield using the percent yield formula. So, you find that 81.37% is the percent yield.
Ques. Define Theoretical Yield Formula. (1 mark)
Ans. Theoretical yield formula is the maximum possible mass of a product that can be made in a chemical reaction. It can begin with a balanced chemical equation by defining the limiting reactant in the process.
Ques. What is Percentage Yield Formula? (1 mark)
Ans. Percent yield formula in a chemical reaction can be calculated by dividing the actual yield by the theoretical yield, and then by multiplying it by 100. It can be denoted by the formula: (Actual Yield / Theoretical Yield) x 100%.
Previous Year Questions Related to Percentage Yield Formula
- Volume of hydrogen formed at 273K and 11 bar pressure is ... (KCET 2020)
- The number of atoms in 4.25 g of NH3 ... (NEET 1999)
Read More:
Read Also:





Comments