
Exams Prep Master
Mass number is defined as the sum of the numbers of protons and neutrons, together known as nucleons, in an atomic nucleus in nuclear physics. It is also called atomic mass number or nucleon number.
- An atomic number of X = Mass number of X - Number of neutrons.
- Mass number and atomic mass number are two of the most important terms used in chemistry.
- When two different elements possess the same mass number, they are called isobars.
Also read: Subatomic Particles of an Atom
| Table of Content |
Keyword takeaways: Mass number, Atomic mass number, Atom, Protons, Neutrons, Electrons, Nucleons, Atomic Mass, Periodic Table
Read More: Number of Moles Formula
Atomic Mass Number
[Click Here for Sample Questions]
The atomic mass number of an element refers to the total number of protons present in the atom of an element. The protons are said to reside in the nucleus of an atom along with the neutrons. So, it can be said that the atomic mass number of an atom is deduced by the number of protons present in its nucleus. Simply, it can be written as atomic mass number = the total number of protons present in the atom.
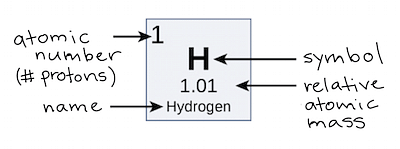
- For instance, suppose an element with 6 protons in its atom.
- So the atomic mass number of the provided element is 6.
- In the same way, Hydrogen with just a single proton in its atom gives it an atomic mass number, of 1.
- The atomic mass number of an element is denoted by 'Z'.
Moreover, every individual element has a different number of protons which provides them with identical atomic mass numbers of their own. The nuclei of all the atoms of an element will have the same number of protons and hence the same atomic mass number. Different elements will have different atomic mass numbers, none can have the same atomic mass number. And atomic mass numbers can be used for identifying different elements as each has got its own identical atomic mass numbers.
Also Read:
| Related Articles | ||
|---|---|---|
| Electron Emission | Classification of Matter | Bond Enthalpy |
| De Broglie Relationship Important Notes | Periodic Classification of Elements | First 20 Elements |
Notation and Importance of Atomic Mass Number
Notation: The atomic mass number of an element is denoted as the subscript on its lower left side. Consider an element X with Z number of protons or the atomic mass number, it will be represented as,
Importance:
- The atomic mass number can be used to identify an element as every element has its own identical atomic mass number.
- The chemical properties of any element are determined by the number of electrons present in it, which in turn is determined by the number of protons or the atomic mass number of the given element.
- It is further helpful in the arrangement of the elements in the periodic table.
Also read: Alpha Particle Scattering and Rutherford’s Nuclear Model of Atom
Mass Number
[Click Here for Previous Year Questions]
The mass number is the total sum of the number of protons and the number of neutrons (or the total number of nucleons) present in an atom. As the mass of electrons is negligible in comparison to that of neutrons and protons, the mass number is calculated using the latter ones. Simply, it can be written as The mass number = total number of nucleons.
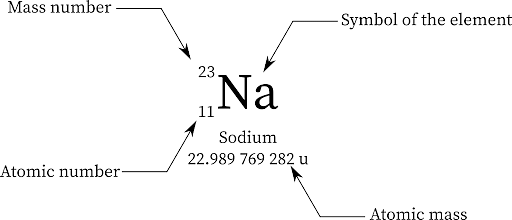
For instance, an atom with 13 protons and 15 neutrons will have 13+15= 28 as its mass number. In the same way, consider an atom of sodium has 12 neutrons and 11 protons, which means its mass number is 12+11 which is 23. The mass number of an element is denoted by 'A'.
Solved ExamplesQues: What do you mean by the mass of hydrogen in terms of amu?
Ans: d) 1.0080 amu Explanation: The mass of a hydrogen atom is 1.6736×10-24 g, which when converted in terms of amu, 1.6736×10-24g should be divided by 1.66056×10-24 g. 1.6736×10-24 g/1.66056×10-24 g = 1.0078 amu = 1.008 amu. This is how any atomic mass in amu is measured. Ques: Nowadays, “amu” is replaced by ____
Ans: a) u Explanation: Nowadays, “amu” is replaced by “u”. The Atomic Mass Unit used to be represented by “amu,”. However, it was changed to “u” and is now called Unified Mass. The mass of one nucleon will be one unified atomic mass unit, which is also equal to 1 g/mol. |
Notation and Importance of Mass Number
Notation: The mass number of an element is denoted as the superscript on its upper left side. Consider an element X with A as its mass number, it will be represented as,
Importance:
- The mass number of elements is also supposed to tell their atomic mass. The reason being the atomic mass of an atom is numerically equal to the mass number of that particular atom.
- It denotes the total mass of the atom of the element.
Note: The mass number and atomic mass number are related as,
The Mass number = The Atomic mass number + the total number of neutrons
Also, both the mass number and the atomic mass number can be written on the left upper and lower sides of the element symbol.

Atomic Number Orbital Energy Levels
[Click Here for Sample Questions]
It is more likely to be found in some portions of that level than others when an electron is at a specific energy level. Orbitals are the name for these domains. Sub-levels are made up of orbitals with the same energy, wherein a maximum of two electrons can be found in each orbital.
- In order to write down the numbers of electrons in each energy level, the atomic number of an element shows how many electrons are there in the atoms.
- For instance, the atomic number of carbon is 6 giving us six electrons as 2,4.
- Therefore, an atom with the atomic number 12 has an electronic structure 2, 8, 2, with two electrons in the inner energy level.
- Eight in the next energy level, and two in the outer highest energy level.
Also Read:
Things to Remember
- The atomic mass number is equal to the total number of protons present in the atom. (A=P)
- The atomic mass number is denoted by 'A'. and it can be written as atomic mass number = the total number of protons present in the atom.
- It is useful in identifying an element as all the atoms have identical atomic mass numbers.
- The atomic mass number can be used to identify an element as every element has its own identical atomic mass number.
- The mass number is equal to the sum of protons and neutrons (or nucleons) present in the atom.
- The mass number is denoted by 'Z'. and it can be simply written as, The mass number = total number of nucleons.
- The mass number and the atomic mass number are related as, Z = A + n.
Previous Years’ Questions
- Electronic configuration of calcium atom can be written as..[NEET 1992]
- If the energies of the two photons are in the ratio of 3 : 2, their wavelengths will be in the ratio of...[KCET 2011]
- Among the elements from atomic number 11 to 36, the number of elements which have an unpaired electron in their s subshell is...[KCET 2014]
- Uncertainty principle is valid for...[KEAM]
- The energy of an electron in the 3S orbital (excited state) of H - atom is...[KEAM]
- The line of longest wavelength corresponds to n2=3...[KEAM]
- Which of the following is the correct unit of angular momentum of an lectron in an orbital of an atom?..[KEAM]
- The momentum of a particle having a de-Broglie wavelength of 1017 m is (Given, h = 6.625×10−34 m)...[NEET 1996]
- The orbitals are called degenerate when:….[NEET 1996]
- Calculate the energy in joule corresponding to light of wavelength 45 nm...[NEET 2014]
- For all gases, at any given pressure, the graph of volume vs temperature (in celsius) is a straight line. This graph is called...[COMEDK UGET 2014]
- What will be the longest wavelength of energy required to remove an electron from the third orbit?..[JKCET 2007]
- Which electronic level would allow the hydrogen atom to absorb a photon but not to emit a photon?..[JEE ADVANCED 1984]
- What is the maximum number of emission lines obtained when the excited electron of a hydrogen atom in n=5 drops to the ground state?..[JKCET 2010]
- Which of the following does not characterise X-rays ?..[JEE ADVANCED 1992]
Sample Questions
Ques: Consider an element X with a total number of protons as 12. What will be its atomic mass number? (1 mark)
Ans: As we know, the total number of protons in an element is equal to the total atomic mass number of the atom. Hence, the atomic mass number will be 12.
Ques: What is meant by atomic mass number? (4 marks)
Ans: The atomic mass number of an element refers to the total number of protons present in the atom of an element. The protons are said to reside in the nucleus of an atom along with the neutrons. So, it can be said that the atomic mass number of an atom is deduced by the number of protons present in its nucleus. Simply, it can be written as atomic mass number = the total number of protons present in the atom. For instance, suppose an element with 6 protons in its atom. So the atomic mass number of the provided element is 6. In the same way, Hydrogen with just a single proton in its atom gives it an atomic mass number, 1.
Ques: Consider an element A with the total number of protons as 9 and the number of neutrons as 12. Calculate its mass number. (1 mark)
Ans: As we already know,
The mass number = total number of protons + total number of neutrons.
So, The mass number = 9+12 = 21.
Ques: Consider the following data and predict the element. (2 marks)
(I) The number of neutrons in its atom is 10.
(II) The total mass number is 22.
Ans: In order to predict the element we need to know the atomic mass number.
As we know, the mass number = total atomic mass number + total number of neutrons
From this, the atomic mass number = the mass number – total number of neutrons
= 22 – 10 = 12
The atomic mass number = 12.
The element with atomic mass number 12 is Magnesium.
Ques: Consider an element with the number of protons as 7 and the number of electrons as 7 in its atom. (2 marks)
(I) Calculate the atomic mass number.
(II) Predict the charge on the atom.
Ans: I) The atomic mass number is equal to the number of protons. So, the atomic mass number is 7.
II) The charge in the atom will be zero as the number of protons and neutrons are the same.
Ques: Let there he and atom with its atomic mass as 6u and the number of protons as 2. (2 marks)
(I) Calculate its mass number.
(II) Calculate the number of protons.
Ans: I) The mass number is equal to the atomic mass. Hence, the mass number is 6.
II) As we know the mass number and the number of protons, using this data we can easily calculate the number of neutrons.
The mass number = number of protons+ number of neutrons
6 = 2+ n
n = 6-2 = 4.
Ques: An element X has 23 as its mass number and 12 as its atomic mass number. Represent this on the symbol of the element. (2 marks)
Ans: As we know the mass number is denoted at the upper left corner of the symbol and the atomic mass number is denoted at the lower-left corner of the symbol. The representation will be as follows:

Ques: An element has 16 protons and 12 neutrons in its atom. Calculate the mass number of its atom. (1 Mark)
Ans: The mass number = total number of protons+ total number of neutrons
= 16+12 = 28.
Hence, the mass number is 28.
Ques: What is meant by mass number? (3 marks)
Ans: The mass number is the total sum of the number of protons and the number of neutrons (or the total number of nucleons) present in an atom. As the mass of electrons is negligible in comparison to that of neutrons and protons, the mass number is calculated using the latter ones. Simply, it can be written as, the mass number = total number of nucleons
For instance, an atom with 13 protons and 15 neutrons will have 13+15= 28 as its mass number. In the same way, consider an atom of sodium has 12 neutrons and 11 protons, which means its mass number is 12+11 which is 23. The mass number of an element is denoted by 'A'.
Ques: What is the molecular mass of carbon dioxide? (2 marks)
Ans: Carbon comprises an individual mass of 12 amu. Oxygen has a mass of 16 amu. The chemical formula for carbon dioxide is CO2, where there is one carbon and two oxygens. 12 (M.wt. of carbon) + 2 x 16 (M.wts. of oxygen) = 12 + 32 = 44. As a result, carbon dioxide has a molecular mass of 44.
Ques. What is the formula mass of NaCl? (2 marks)
Ans. The formula mass of Cl can only be calculated because it can not exist in the solid state of NaCl. Sodium comprises a mass of 23 units, and chlorine has a mass of 35.5 units. Individual masses (23 + 35.5 = 58.5 u) are added together in the formula mass of Sodium chloride.
Do Check Out:





Comments