
Content Strategy Manager
Dysprosium (Dy) is a chemical element abundantly found in nature. It is an earth metal of the periodic table and belongs to the lanthanide group. It falls under the 6th period and f block. In 1886, a French chemist named Paul-Émile Lecoq de Boisbaudran found the metal associated with holmium and other heavy lanthanides. Later, in the year 1906, Georges Urbain, a French chemist was able to prepare a reasonably pure extract of Dysprosium. The sources of Dysprosium are predominantly naturally recurring minerals such as laterite ionic clays gadolinite, xenotime, euxenite, fergusonite, blomstrandine, and polycrase.
| Table of Content |
Key Terms: Dysprosium, Lanthanides, Alkaline earth-metals, Ores, Vacuum Distillation, Dysprosium nitrate
Properties of Dysprosium
[Click Here for Sample Questions]
The important properties of Dysprosium are tabulated below:
| Atomic Number | 66 |
| Atomic Weight | 162.5 |
| Melting Point | 1412°C |
| Boiling Point | 2567°C |
| Density | 8.551 gm/cm³ |
| Oxidation State | +3 |
| Electronic Configuration | [Xe]4f106s² |
Also Read:
| Related Articles | ||
|---|---|---|
| Inner Transition Elements | Lanthanum | Actinoids |
| Group 15 elements | Dinitrogen | Oxides of Nitrogen |
| Group 16 elements | Dioxygen | Sulphur dioxide |
Extraction of Dysprosium
[Click Here for Sample Questions]
Dysprosium is primarily obtained from monazite sand, which is a phosphate mixture. The material is obtained as a byproduct of nuclear fission and commercial yttrium extraction. Most undesired metals can be excluded magnetically or through a flotation process when isolating dysprosium. Liquid-liquid extraction or ion-exchange methods are used in the commercial separation of dysprosium from its ores. The metal is produced through the metallothermic reduction of anhydrous halides with alkali or alkaline-earth metals. The resulting dysprosium ions can then react with either fluorine or chlorine to form fluorides and chlorides. These compounds can be reduced using either calcium or lithium metals as follows:
3 Ca + 2 DyF3 \(\rightarrow\) 2 Dy + 3 CaF2
3 Li + DyCl3 \(\rightarrow\) Dy + 3 LiCl
Vacuum distillation is used to purify the metal obtained from the separation of ores. Dysprosium is categorized into 3 allotropic forms. At room temperature, the close-packed hexagonal α-phase has a stable structure. At -363.15°C, dysprosium becomes a hexagonally close-packed lattice with ferromagnetic properties called β-Dy. The γ-phase of dysprosium at 1,381 °C is characterized by body-centered cubic structural forms. Dy was transformed into a 2-dimensional supersolid quantum gas in 2021.

Dysprosium
Physical and Chemical Properties of Dysprosium
[Click Here for Sample Questions]
Dysprosium is a hard metal that has a silvery-white lustrous appearance in its pure form.
It is stable and is a solid-state at room temperature. Dysprosium turnings are easily flammable and burn white in colour. The dysprosium dissolves quickly in diluted acids except for hydrofluoric acid (HF), with which it forms an insoluble trifluoride compound of the protective layer.

Properties of Dysprosium
It exhibits varied properties in various temperatures. Dysprosium is a strong paramagnet above 180 K. It is antiferromagnetic between about 90 and 180 K while acts as a ferromagnetic metal below 90 K.The naturally occurring isotopes are all stable and have mass numbers ranging between 156 to 164. Leaving out the nuclear isomers, a total of 29 radioactive isotopes of dysprosium are observed to date, with mass numbers ranging from 138 to 173.
The least stable is dysprosium-139 and has a half-life of 0.6s, while the most stable is dysprosium-154 has a half-life of 3.0 × 106 years. The earth's crust has approximately 5.2 mg/kg of Dy, and seawater has a concentration of 0.9 mg/L dysprosium. The global production of Dysprosium (Dy) is nearly 100 tonnes, and almost the entire artificial production of dysprosium traces back to China. Dysprosium behaves chemically as a typical trivalent rare earth, forming a series of pale yellow compounds with an oxidation state of +3.
Uses of Dysprosium
[Click Here for Sample Questions]
Dysprosium can be used for a number of purposes like:
- Because of its ability to absorb neutrons, dysprosium is used to control rods in nuclear power plant reactors.
- Because it is a radioactive element, the metal has applications in the field of radioactivity.
- Because of its high magnetic susceptibility, it is used in data storage system applications.
- For high-intensity lamps, Dy bromide and iodide are used.
- In adiabatic refrigerators, Dysprosium salts such as Aluminium Garnet and Iron Garnet are used.
- Dysprosium is used in the production of laser materials and commercial lighting, along with vanadium and other elements.
- This material is used in transducers, wide-band mechanical resonators, and high-precision liquid-fuel injectors.
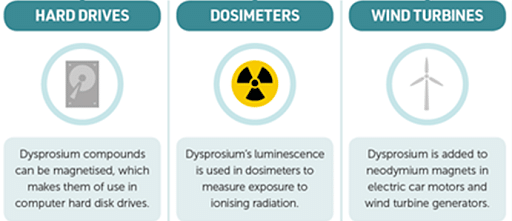
Uses of Dysprosium
Precautions
[Click Here for Sample Questions]
When mixed with air and an ignition source, dysprosium powder can cause an explosion. Sparks or static electricity can also ignite thin foils of this substance. Water cannot extinguish a dysprosium fire as it reacts slowly to water. It can combine with water to form flammable hydrogen gas. But water can be used to extinguish fires caused by dysprosium chloride.
Dysprosium fluoride and oxide are not flammable in nature. Dysprosium nitrate is a strong oxidizing agent that easily ignites when it comes into contact with organic materials.
Soluble dysprosium salts such as dysprosium chloride and dysprosium nitrate are mildly toxic. Based on the toxicity of dysprosium chloride to mice in an experiment, it is estimated that ingesting 500 grams or more of the Dy could be fatal to humans. The insoluble salts on the other hand are not poisonous.
Things to Remember
- Dysprosium belongs to the lanthanide series of earth metals. It is not found in a free state naturally.
- It has a silvery-white appearance and is easily flammable.
- Dysprosium has an atomic number of 66 and atomic weight of 162.5 amu.
- With lower temperature it exhibits ferromagnetic properties and dissolves in dilute acids.
- Consuming more than 500 grams of Dy can be fatal for humans.
Also Read:
Sample Questions
Ques. What group does Dysprosium belong to? (2 Marks)
Ans. Dysprosium belongs to the Lanthanide series. It is the 66th element of the periodic table and falls under the 6th period and f block. The atomic weight of the Dysprosium is 162.5 amu. It was discovered with holmium and other heavy lanthanide metals.
Ques. What are the processes involved in the extraction of Dysprosium commercially? (3 Marks)
Ans. Liquid-liquid extraction or ion-exchange methods are used in the commercial separation of Dysprosium from its ores. The metal is produced through the metallothermic reduction of anhydrous halides with alkali or alkaline-earth metals. The resulting dysprosium ions can then react with either fluorine or chlorine to form fluorides and chlorides.
3 Ca + 2 DyF3 \(\rightarrow\) 2 Dy + 3 CaF2
3 Li + DyCl3 \(\rightarrow\) Dy + 3 LiCl
Vacuum distillation is further used to purify the metal obtained.
Ques. Give five chemical properties of Dysprosium. (5 Marks)
Ans. The chemical properties of dysprosium are:
- Dysprosium is a strong paramagnet above 180 K.
- Dysprosium nitrate is a strong oxidizing agent that easily ignites when it comes into contact with organic materials.
- Its melting point is 1412°C and its boiling point is 2567°C.
- It has a total of 29 radioactive isotopes.
- Dysprosium behaves chemically as a typical trivalent rare earth, forming a series of pale yellow compounds with an oxidation state of +3.
Ques. Give five physical properties of Dysprosium. (5 Marks)
Ans. The physical properties of dysprosium are:
- Dysprosium is a hard metal with a silvery-white lustrous appearance in its pure form.
- It is not found in a free state and pure form is obtained through artificial processing.
- It is a stable solid at room temperature.
- Dysprosium turnings are easily flammable and burn white in colour.
- The Dysprosium dissolves quickly in diluted acids except for HF.
Ques. What are the applications of Dysprosium? (5 Marks)
Ans. The applications of dysprosium are:
- With its ability to absorb neutrons, dysprosium is used to control rods in nuclear power plant reactors.
- As a radioactive element, the metal has applications in the field of radioactivity.
- Because of its high magnetic susceptibility, it is used in data storage system applications.
- For high-intensity lamps, Dy bromide and iodide are used.
- In adiabatic refrigerators, Dysprosium salts such as Aluminium Garnet and Iron Garnet are used.
Ques. List three dangers associated with Dy? (3 Marks)
Ans. The dangers associated with dysprosium are:
- When mixed with air and an ignition source, dysprosium powder can cause an explosion.
- Even thin foils ignite with a spark or static electricity.
- Soluble dysprosium salts such as dysprosium chloride and dysprosium nitrate are mildly toxic and 500 grams or more is fatal for human beings.
Ques. What are the allotropic forms of Dysprosium? (3 Marks)
Ans. Dysprosium has 3 allotropic or structural forms:
- The α-phase, is a close-packed hexagonal stable structure at room temperature.
- At -363.15°C, Dysprosium becomes a hexagonally close-packed lattice with ferromagnetic properties called β-Dy.
- The γ-phase of Dysprosium at 1,381°C has a body-centered cubic structural form.
Ques. State the behaviour of Dysprosium under varied temperatures. (2 Marks)
Ans. It exhibits varied properties in various temperatures. Dysprosium is a strong paramagnet above 180 K. It is antiferromagnetic between about 90 and 180 K while acts as a ferromagnetic metal below 90 K.
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Check-Out:





Comments