
Content Curator
Isotopes are elements which have the same atomic number but different atomic mass. Isobars are those where the elements have different atomic numbers but the same atomic mass. Property of Isotopes usually occur in the same element whereas property of Isobar is seen in different elements.
- Example of Isotopes: Hydrogen has 3 isotopes namely, Protium (1H1), Deuterium (1H2), Tritium (1H3).
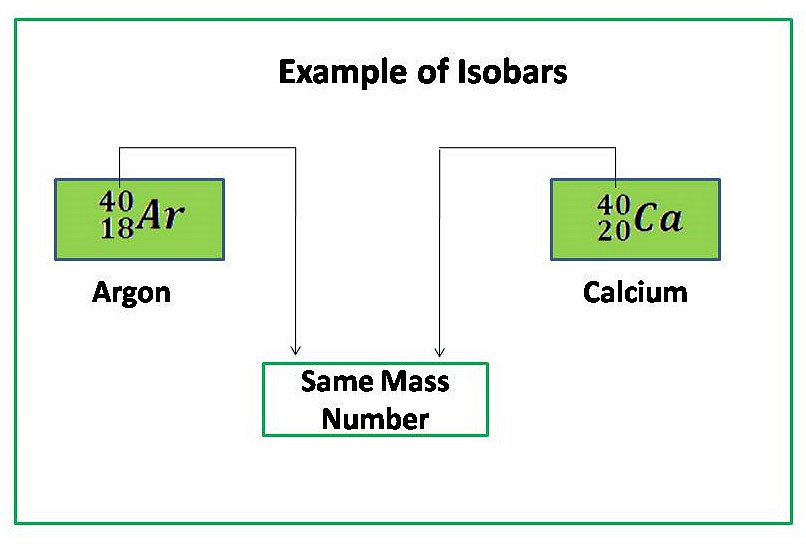
- Example of Isobars: Argon (18Ar40), Potassium(19K40) and Calcium(20Ca40) have the same atomic mass.
Read More: Isotopes of Hydrogen
| Table of Content |
Isotopes
[Click Here for Sample Questions]
- Isotopes are elements that have the same atomic numbers but different mass numbers.
- Atomic number is the number of protons or electrons in an atom (Number of protons = Number of electrons).
- Mass number is the sum of the total mass of protons and neutrons.
- As the mass of electrons is negligible, it is not considered.
- Isotopes are atoms in which the number of neutrons differs from one another but the number of protons does not.
Example of Isotopes
- Protium, deuterium, and tritium are the three hydrogen isotopes.
- They all have the same amount of protons, however, the number of neutrons varies.
- Protium has 0 neutrons, deuterium has 1 neutron and tritium has 2 neutrons.

Also Read:
| Related Articles | ||
|---|---|---|
| Difference between Atom and molecule | Ions | How to find Atomic Mass |
| How do atoms exist? | What is a molecule? | Writing Chemical Formula |
Isobars
[Click Here for Previous Year Questions]
- Isobars as elements having different atomic numbers but the same mass number.
- Isobars are elements that have different chemical properties but the same physical properties.
- Isobars have the same atomic mass but a different atomic number because the discrepancy in the number of nucleons is compensated by an increased number of neutrons.
- Although the number of protons and neutrons will vary, the number of nucleons, or the sum of protons and neutrons in isobars, will remain constant.
- Because of the difference in atomic numbers, isobars always have different atomic structures.
- For example, Iron and nickel are isobars. The atomic numbers of iron and nickel are 26 and 28, respectively. The mass number of both is the same i.e. 58.
Examples of Isobars
- Ar, K and Ca
- Ce and Se
- Na and Mg
- Fe and Ni
- Co and Ni
Difference Between Isotope and Isobar
[Click Here for Sample Questions]
Key differences between Isotope and Isobar are tabulated below.
| Isotope | Isobar |
|---|---|
| Isotopes are atomic structures of the same elements having a different mass number/atomic mass | Isobars are different chemical elements having the same atomic mass. |
| Atomic numbers of all isotopic forms of a single element are equal. | Atomic numbers of isobars vary from each other |
| They are the same chemical element but their forms are different | They are different elements altogether. |
| All isotopic forms of single elements have different physical properties. | Physical properties can be similar to each other |
Also Read:
| Related Articles | ||
|---|---|---|
| Mole concept | Atomic Spectra | Avogadro’s Number |
| Alpha particle mass | Law of Chemical Combination | Intermolecular Forces |
Uses of Isotopes
[Click Here for Previous Year Questions]
Isotopes have a variety of uses which are given below.
Medical Applications of Isotopes
- Cobalt-60 emits gamma rays, which can be used in cancer treatment via radiotherapy.
- Less penetrating radiation from strontium-90 or phosphorus-32 is used to treat superficial tumors like skin cancer.
- A heart pacemaker containing plutonium-238 can be used to help individuals with heart issues regulate their heartbeats.
- Thyroid illness can also be treated with iodine-131.
- Carbon-14 is used to determine the age of bones, fossils, and wood by measuring the carbon-14 content.
Isotope Uses in Agriculture
- Using a phosphate fertilizer containing phosphorus-32, the phosphate and metabolic absorption of phosphorus by plants can be studied.
- Furthermore, research utilizing carbon -14 as a radioactive tracer has aided in the understanding of protein production and photosynthesis.
Isotope Uses in the Industry
- Sodium-24 is used to locate gas or oil pipeline breaches, as well as ventilation systems.
- Krypton-85 radiation is used to manage the thickness of plastic sheets.
- The gamma rays of Cobalt-60 are passed into food to kill microorganisms, causing food to spoil without compromising its flavor, quality, or texture.
Uses of Isobars
[Click Here for Sample Questions]
- The isobars of uranium can be used in nuclear reactors.
- Isobars of iodine are used to treat goiter.
- Isobars of cobalt can be used to treat cancer.
Also Read:
| Related Articles | ||
|---|---|---|
| Metals and Non Metals | Carbon & its Compounds | Chemical Reactions and Equations |
| Difference between elements and compounds | Spectral Series | Bohr Radius |
Things to Remember
- Mass number is the sum of protons and the number of neutrons.
- Isobars have different atomic numbers but same mass number.
- Isobars are elements that have different chemical properties but same physical properties.
- Isotopes have similar atomic numbers but different mass numbers.
- Isotopes are elements that have same chemical properties but different physical properties.
Previous Year Questions
|
Sample Questions
Ques: What is an isotope? (2 Marks)
Ans: The atoms of the same element which have same atomic number but different mass number are called isotopes.
Example: Isotope of Chlorine- 17Cl35 and 17Cl37
Isotope of Hydrogen- 1H1 ,1H2 and 1H3
Ques: What are isobars? (2 Marks)
Ans: Atoms of different elements which have the same mass number but different atomic number are called isobars.
Example: 6C14 and 7N14
18Ar40 ,19K40 and 20Ca40
Ques: Two atoms are said to be isobars if. (1 Mark)
(i) they have same atomic number but different mass number.
(ii) they have same number of electrons but different number of neutrons.
(iii) they have same number of neutrons but different number of electrons.
(iv) sum of the number of protons and neutrons is same but the number of protons is different.
Ans: (iv) sum of the number of protons and neutrons is same but the number of protons is different.
Ques: Chlorine exists in two isotopic forms, Cl-37 and Cl-35 but its atomic mass is 35.5. This indicates the ratio of Cl-37 and Cl-35 is approximately (1 Mark)
(i) 1:2
(ii) 1:1
(iii) 1:3
(iv) 3:1
Ans: (iii) 1:3 is the correct answer.
Ques: Identify the pairs which are not of isotopes? (1 Mark)
(i) 1H1, 1H3
(ii) 6C12 , 6C13
(iii) 8O16, 9O16
(iv) 20Ca40, 21Ca40
Ans: Option (iii) and (iv) are the correct answers.
Ques: In which of the following pairs, the ions are iso-electronic? (1 Mark)
(i) Na+, Mg2+
(ii) Al3+, O–
(iii) Na+, O2-
(iv) N3–, Cl–
Ans: (i) Na+, Mg2+ and (iii) Na+, O2-
Ques: Why the Elements Have Isobars and Isotopes? (1 Mark)
Ans: Elements have distinct isotopes due to the existence of a varying number of neutrons in atoms of the same element. Due to the identical mass number (s) of neutrons and protons, various elements might have distinct isobars.
Ques: How Do the Isotopes Form? (1 Mark)
Ans: Isotopes are atoms that have the same proton number but a varying number of protons and neutrons.
Ques: Explain Isotopes and Isobars with Examples? (2 Mark)
Ans: Isotopes have the same number of protons but differ in the number of neutrons they have. “Isotopes possess the same number of Protons,” says the pneumonic. For example, the isotopes are carbon-12, carbon-13, carbon-14, and carbon-15.
The nucleons and atomic mass of the isobars are the same, but the number of protons and neutrons is different. Isobars have a comparable Atomic mass. For example, isobars boron-12, oxygen-12, nitrogen-12, and carbon-12 exist.
Ques: Give the difference between isobars and isotopes. (2 Mark)
Ans: The difference between isobars and isotopes is given below:
| Isobars | Isotopes |
|---|---|
| Isobars are different chemical elements having the same atomic mass. | Isotopes are atomic structures of the same elements having a different mass number/atomic mass |
| Atomic numbers of isobars are different from each other. | Atomic numbers of all isotopic forms of a single element are equal. |
| They are different elements altogether. | They are the same chemical element but their forms are different |
| Physical properties can be similar to each other | All isotopic forms of a single element have different physical properties |
For Latest Updates on Upcoming Board Exams, Click Here: https://t.me/class_10_12_board_updates
Do Check Out:





Comments