
Content Curator
Atomic number is denoted by the number of protons or electrons present in the atom. Atomic number basically determines the type of element we are looking at. Protons, neutrons, and electrons make up atoms, which are the fundamental building units of matter. The amount of positively charged protons must be equal to the number of negatively charged electrons in an atom since it is electrically neutral. Neutrons have no influence on a charge, and therefore they remain electrically neutral. But neutrons can vary even amongst atoms of the same element.
Read about: Proton Mass
Keyterms: Atoms, protons, neutrons, electrons, nucleus, oxygen, modern periodic table, nuclear physics
Atomic Number
[Click Here for Sample Questions]
The number of protons in the nucleus of each atom of an element is denoted by its atomic number (represented by the symbol Z). Based only on its atomic number, an atom can be classed as a certain element. Any atom with an atomic number of 8 (with an 8-proton nucleus) is an oxygen atom, while an atom with a different number of protons is a different element. The modern periodic table is arranged based on the atomic number of the elements.
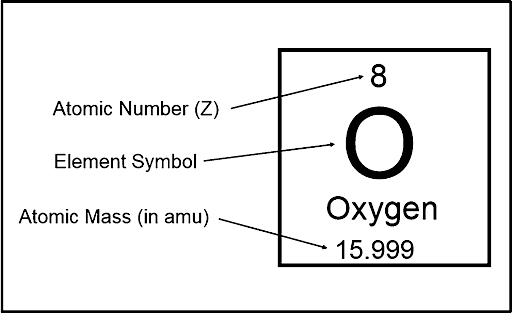
Atomic number
The number of electrons in an atom equals the number of protons since atoms are neutral. The region outside the nucleus of hydrogen atoms is occupied by one electron and the nucleus has only one proton and no nucleus. Helium will contain two electrons and two protons. The proton count will always be equal to the atomic number of an atom. If the nucleus decays or gets bombarded, this value will not change (nuclear physics).
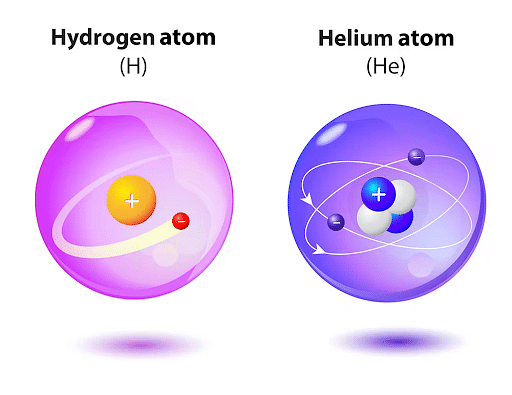
Hydrogen and Helium
Representation of Atomic Number
[Click Here for Previous Year Questions]
Atomic number is represented by the letter Z. Periodic table is arranged based on the ascending order of the element’s atomic number.

Representation of Atomic Number
The mass number of the element is denoted by the letter A. Mass number is the total mass of the atom, i.e, the mass of protons and neutrons.
Read More: Energy Level Diagram
Atomic Numbers' Importance
[Click Here for Sample Questions]
- Assists in identifying a certain atom's element.
- It is the foundation for the periodic table's arrangement of elements. The elements are listed in ascending atomic number order.
- Any element's attributes can be determined with the use of this tool. However, the chemical bonding behaviour of an element is determined by the valence electron.
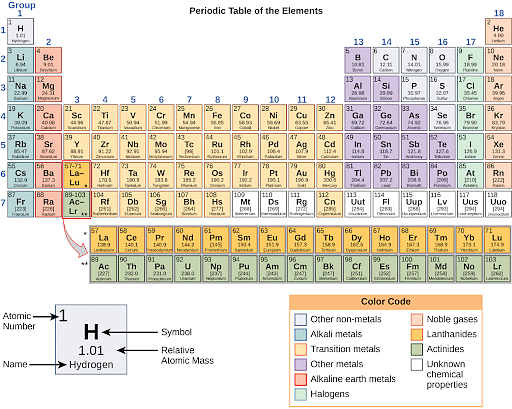
Periodic Table
Also Read:
| Related Articles | ||
|---|---|---|
| Group 15 Elements | Group 16 Elements | Group 18 Elements |
| Homogeneous Equilibrium | Phase Changes | Arrhenius Equation |
| Solutions | Types of Solutions | Solubility |
Atomic Mass
[Click Here for Previous Year Questions]
The mass number of an atom is equal to the number of protons and neutrons combined. The letter 'A' is used to symbolize it. Nucleons are made up of protons and neutrons, which are found in the nucleus of an atom.
For example, Carbon has six protons and six neutrons. It has a mass of 12. The number of protons in an element's atoms remains constant, whereas the number of neutrons varies. As a result, differing mass numbers can exist among atoms of the same element, which are referred to as isotopes. An electron's mass is extremely little. As a result, an atom's atomic mass and mass number are nearly equal.

Isotopes of Hydrogen
Take helium as an example. It possesses two protons in its nucleus, as its atomic number is 2. There are two neutrons in its nucleus as well. The mass number of a helium atom is 4 since 2+2=4. Finally, because the number of electrons must equal the number of protons, the helium atom likewise includes two electrons. This example might lead you to conclude that atoms have the same number of protons and neutrons, but a closer look at the periodic table reveals that this is not the case. For instance, lithium has three protons and four neutrons, giving it a mass number of seven.
By subtracting the mass number and the atomic number of an atom, you can calculate the number of neutrons present in that atom.
Number of neutrons = mass number (rounded) − atomic number
Read More: Orbitals
Periodic Table
[Click Here for Sample Questions]
As new elements were discovered, researchers looked to see whether there was any similarity or consistency in the information provided. Mendeleev attempted to systematize these facts early on, which led to the discovery of the first periodic table. The early periodic table, which was based on atomic weights, was critical in revealing new element identities. Mendeleev upgraded his periodic chart based on the number of protons present in an atom's nucleus, i.e., the atomic number, after discovering the nucleus.

Mendeleev Periodic Table based on Mass Number
- Additionally, elements are grouped in horizontal rows with increasing atomic numbers. Periods are the rows and Groups are the columns in the table
- The greatest number of elements a period may hold, is determined by the number of electrons in the atom's outermost shell.
- The total number of shells contained in an element's atom is represented by its period number.
- The electrons are gradually added to the shells as you go over a period from left to right.
- When the outermost shell has eight or eighteen electrons, the elements move on to the next phase.
The elements in the modern periodic table are listed in order of increasing atomic number. The number of protons determines the number of electrons surrounding the nucleus, which is determined by the atomic number.
Group 1A elements, for example, are all soft metals with 1+ charges that react aggressively with water. At room temperatures, all Group 8A elements are monoatomic gases that are stable and unreactive. The arrangement of these electrons controls the majority of an element's chemical behavior. As a result, an element's atomic number is critical to its identification and chemical properties.
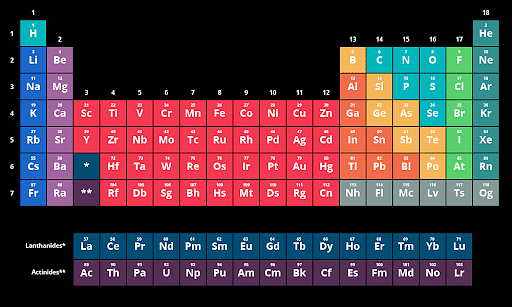
Periodic Table
Read about: Periodic properties of elements
Valency
[Click Here for Previous Year Questions]
The valency of an atomic orbit is the number of unpaired electrons in the outer shell. The valency of an element is the last unpaired electron in an electronic configuration. The atomic number of sodium, for example, is 11, and the valency is determined by the number of last unpaired electrons, with the unpaired electron numbering one. As a result, sodium has a valency of 1.

Valence Electrons of Sodium
The letters K, L, M, N, and so on stand for the orbitals (shells) in which electrons in an atom are arranged. The electrons in an atom's outermost shell/orbit are known as valence electrons. Valence electrons play a role in any chemical reaction because the outermost orbit usually contains more energy than the electrons in other orbits.

Orbitals of Chlorine
According to the Bohr-bury system, an atom's outermost orbit can include up to 8 electrons. The element, on the other hand, has very little to no chemical activity when the outermost orbit is completely filled. Their ability to combine becomes insignificant or non-existent. Because their outermost orbit is completely filled, noble gases are the least reactive. Other elements' reactivity, on the other hand, is determined by their ability to form noble gas configurations. It will also aid in determining an atom's valency.
Also Read:
Things to Remember
- The atomic number of a chemical element is determined by the total number of protons in its nucleus. An atomic number is a number that identifies each element. The letter "Z" is a symbol for it.
- The total number of protons and neutrons (often referred to as nucleons) in an atomic nucleus is represented by the mass number (symbol A).
- The tendency of an atom to combine with other atoms is defined by its valency. The number of bonds that an atom can form as part of a complex is determined by the element's valency.
- Nucleons are subatomic particles that make up the nucleus of an atom and are made up of protons and neutrons.
- Because electrons rotate around the nucleus, they are not considered nucleons.
Previous Year Questions
- How many electrons can fit in the orbital for which n = 3 and l = 1 ?...[NEET 2016]
- In 3p-orbital, the number of spherical nodes is...[NEET 1988]
- In hydrogen atom, energy of first excited state is -3.4 eV. Then, KE of same orbit of hydrogen atom :-...[NEET 2002]
- In hydrogen atom, the de Broglie wavelength of an electron in the second Bohr orbit is : [Given that Bohr radius,a0 = 52.9pm ]...[NEET 2019]
- In the photoelectron emission, the energy of the emitted electron is….[NEET 1994]
- What will be the longest wavelength line in Balmer series of spectrum?..[NEET 1996]
- Which one is the wrong statement ?...[NEET 2017]
- Which of the following statements do not form a part of Bohr's model of hydrogen atom?...[NEET 1989]
- Which of the following pairs of d-orbitals will have electron density along the axes?...[NEET 2016]
- Which of the following is never true for cathode rays?..[NEET 1994]
- The wave number of the spectral line in the emission spectrum of hydrogen will be equal to {9}98 times the Rydberg's constant if the electron jumps from _______...[KCET 2010]
- Which of the following statement is true ?..[KCET 2015]
- Which of the particles cannot be accelerated?..[KCET 2005]
- Impossible orbital among the following is...[KCET 2012]
- The electron configuration Is2 2s2 2p6 3s2 3p6 3d{10} represents….[KCET 2006]
- Which one of the following sets of ions represents the collection of isoelectronic species ?...[KCET 2013]
Sample Questions
Ques. When the atomic number is 18 and the neutron number is 20, what is the element's mass number? [2 marks]
Ans. The number of neutrons is equal to 20.
Total number of protons = 18
Number of protons + neutrons = atomic mass number
20 + 18 = A
No. of mass = 38
Ques. What is the mass number of an element with the atomic number of 15 and a total number of protons of 15? [2 marks]
Ans: Using the formula, we can compute the mass number.
The number of protons in an atom is expressed as an atomic number.
Number of Protons + Number of Electrons = Mass Number
15 + 15 = 30
Read More: Clemmensen Reduction Reaction
Ques. What is the method for calculating the valency of an element with the atomic number 51? [2 marks]
Ans. Antimony is a chemical element with atomic number 51. (Sb). The following formula can be used to calculate its valency:
The number 51 is the atomic number.
Electronic Configuration = 2, 8, 18, 18, 5.
There are five electrons in the outermost shell.
As a result, Valency equals 5.
Ques. Why is valency important? [2 marks]
Ans. Valence is important because when two atoms react, their outer shells come into touch first, and it is the outer shell electron that is generally engaged in a chemical reaction. To stabilize their valence shell, atoms exchange valence electrons in a chemical reaction.
Ques. From the atomic number and the number of neutrons, how do you calculate the atomic mass? [2 marks]
Ans. Calculate the atomic mass of a single atom of an element by adding protons and neutrons' masses. The mass number of an element is calculated by adding the number of protons and neutrons together: mass amount = protons + neutrons.
Read More: Tollen’s Test
Ques. What are the characteristics of valence electrons? [4 marks]
Ans. Valence Electrons have the following characteristics:
- Within atoms, electrons can be shared and exchanged, and the octet rule can be satisfied to achieve noble gas configuration.
- The periodic table can be used to determine the number of valence electrons in an atom.
- For main group elements, valence electrons can occur in the outermost shell.
- Valence electrons, on the other hand, can live in a transition element's inner shell.
- If an atom contains a closed shell with valence electrons, it will be chemically alert.
- Photons can be used to release and absorb energy from valence electrons.
- Valence Electrons can be used to determine the electrical conductivity of any element.
- We can tell if it's metal, non-metal, or a metalloid by looking at it.
Ques. With 15 electrons, 15 protons, and 16 neutrons, what element is it? [2 marks]
Ans. An atom with 15 protons has an atomic number of 15, hence the atomic number of the given element is similarly 15. Phosphorus is an element with an atomic number of 15. Phosphorus has a mass number of 31 (atomic number + neutron number).
The element chromium (Cr) has a mass number of 52 and an atomic number of 24. A chromium atom's nucleus contains how many neutrons? To figure this out, subtract as follows:
In a chromium atom: 52 − 24 = 28 neutrons.
Ques. What is the atomic number of an element whose mass number is 23 and contains 12 neutrons in its nucleus? What is the symbol of an element? [2 marks]
Ans. Atomic number = No. of protons in the nucleus
= Mass no. – No. of neutrons
= 23 – 12 = 11
The element is sodium and its symbol is Na.
Ques. The nucleus of an atom has 6 protons and 8 neutrons. What are its atomic number and mass number? What is this element1? [2 marks]
Ans. At. No. = 6;
Mass No. = 6 + 8 = 14
Element is Carbon.
Ques. Define atomic number, mass number and neutron. How are the three related to each other? [5 marks]
Ans. Atomic Number (Z): The atomic number of an element is equal to the number of protons present inside the nucleus of its atoms.
Since an isolated atom has no net charge on it, in neutral atoms, the total number of electrons is equal to its atomic number.
Atomic number (Z) = Number of protons in the nucleus of an atom = Number of electrons in the neutral atoms
Mass Number (A): The sum of the number of neutrons and protons in the nucleus of an atom is called its mass number. Mass number is denoted by A. Thus, for an atom, Mass number (A) = Number of protons (p) + Number of neutrons (n)
A = p + n
Neutron: It is a neutral particle. It is present in the nucleus of an atom. Except for hydrogen (which contains only one electron and one proton but no neutron), the atoms of all other elements including isotopes of hydrogen contain all the three fundamental particles called neutrons, protons and electrons.
The relation between mass number, Atomic no. and no. of neutrons is given by the equation: A= Z + n
Where A = Mass number
Z = Atomic number n = number of neutrons in the nucleus.
Do Check Out:





Comments