
Content Writer
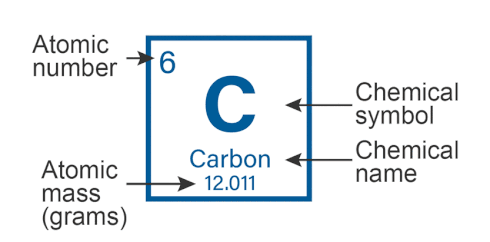
Atomic Mass of Elements is the total mass of one atom of a given element. It is the average mass of protons, neutrons, and electrons in an atom. Atomic Mass is denoted by the letter ‘A’ and gives the average weight of an element in the periodic table.
- The atomic mass of an element is almost the same as its mass number.
- It is measured in Atomic Mass Units (amu) or Daltons.
- Atomic Mass is defined as the weight of 1/12th of the mass of a carbon-12 atom, which is a carbon atom containing 6 neutrons.
- Atomic mass is used in the classification of different isotopes of the same element.
However, elements have different isotopes of distinctive atomic masses and thus a general atomic weight is determined for the element.
Read More: NCERT Solutions For Class 11 Chemistry Some Basic Concepts of Chemistry
Atomic Mass of Elements
[Click Here for Sample Questions]
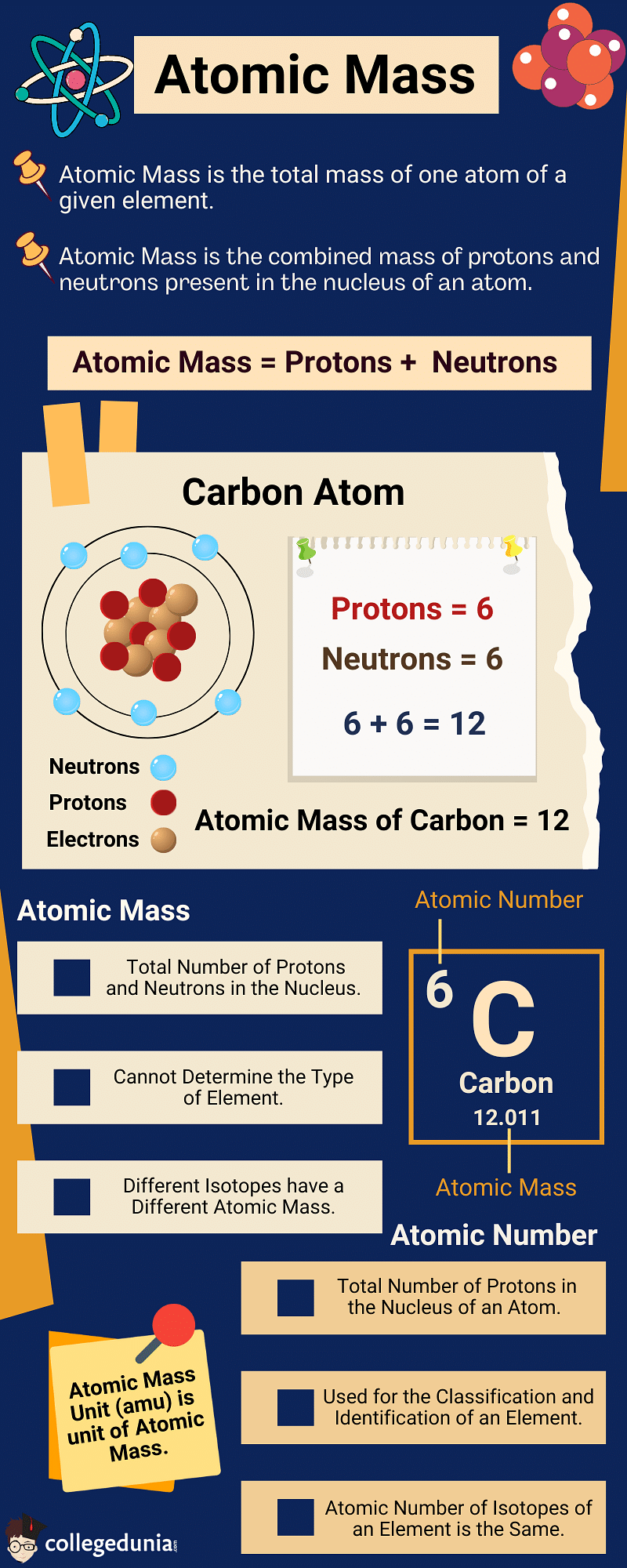
Atomic Mass of an Element is defined as the average mass of the atoms in an element.
- It is the combined mass of protons and neutrons present in the nucleus of an atom.
- It is the one-twelfth mass of a carbon-12 atom which is equal to 1.992646547 × 10-23 grams.
- The unit of atomic mass is Atomic Mass Units (amu).
- Atomic mass is also calculated in the unit of Dalton.
- The average value of the atomic masses in a mixture of isotopes is given by the standard atomic weight of an element.
- Since protons and neutrons make up the entire mass of an atom, the atomic mass of an element is almost equal to its mass number.
Read More:
Atomic Mass of First 30 Elements
[Click Here for Previous Years’ Questions]
Elements are arranged in the modern periodic table according to the basis of their atomic number. The atomic mass of an element is necessary to calculate the atomic mass of a mixture of different elements.
Atomic Mass of First 30 Elements of the periodic table is listed as follows:
| Atomic Number | Element | Atomic Mass |
|---|---|---|
| 1 | Hydrogen | 1.008 |
| 2 | Helium | 4.0026 |
| 3 | Lithium | 6.94 |
| 4 | Beryllium | 9.0122 |
| 5 | Boron | 10.81 |
| 6 | Carbon | 12.011 |
| 7 | Nitrogen | 14.007 |
| 8 | Oxygen | 15.999 |
| 9 | Fluorine | 18.998 |
| 10 | Neon | 20.18 |
| 11 | Sodium | 22.99 |
| 12 | Magnesium | 24.305 |
| 13 | Aluminum | 26.982 |
| 14 | Silicon | 28.085 |
| 15 | Phosphorous | 30.974 |
| 16 | Sulfur | 32.06 |
| 17 | Chlorine | 35.45 |
| 18 | Argon | 39.948 |
| 19 | Potassium | 39.098 |
| 20 | Calcium | 40.078 |
| 21 | Scandium | 44.956 |
| 22 | Titanium | 47.867 |
| 23 | Vanadium | 50.942 |
| 24 | Chromium | 51.996 |
| 25 | Manganese | 54.938 |
| 26 | Iron | 55.845 |
| 27 | Cobalt | 58.933 |
| 28 | Nickel | 58.693 |
| 29 | Copper | 63.546 |
| 30 | Zinc | 65.38 |
Read More: Some Basic Concepts of Chemistry
Atomic Mass
Difference between Atomic Mass and Atomic Number
[Click Here for Sample Questions]
Atomic Mass and Atomic Number are often confused with each other, however, they are used to reveal different characteristics of elements.
- Atomic Number is defined as the number of protons in the nucleus of an atom.
- Atomic Mass is the number of protons and neutrons in the nucleus of an atom.
Here is an example of the 8th element in the periodic table to see the difference between atomic number and atomic mass.

8th Element in Periodic Table
The key difference between Atomic Mass and Atomic Number is as follows:
| Atomic Mass | Atomic Number |
|---|---|
| Atomic Mass is calculated as the total number of protons and neutrons in the nucleus of an atom. | Atomic Number is the number of protons in the nucleus of a given atom. |
| It gives the average weight of an element. | It gives the total number of protons in the atom’s nucleus. |
| It is denoted by the letter ‘A’. | It is represented by the letter ‘Z’. |
| The type of an element cannot be determined using atomic mass. | Atomic number is used for the classification and identification of an element. |
| It is used to differentiate the isotopes of the same element. | The atomic number of isotopes of the same element is the same. |
| It is measured using atomic mass units (amu). | It is just a number used for the placement of elements in a periodic table. |
Read More: Some Basic Concepts of Chemistry Important Questions
How to Calculate Atomic Mass?
[Click Here for Previous Years’ Questions]
The atomic mass of an element is calculated by the combined mass of protons and neutrons. The number of protons is always equal to the atomic number. The number of neutrons is dependent on the isotope of the element.
Atomic Mass Formula is given as
| Atomic Mass = Mass of Protons + Mass of Neutrons + Mass of Electrons |
Solved ExampleExample: Calculate the atomic mass of an oxygen isotope with 9 neutrons. Solution: We know that, Atomic Mass = Number of Protons + Number of Neutrons
Atomic Mass of Given Oxygen Atom = 8 + 9 = 17 |
Mass Number
[Click Here for Sample Questions]
Mass Number is defined as the total number of protons and neutrons in the nucleus of an atom.
- If the nucleus of an atom of carbon contains 6 protons and 6 neutrons, it will have an exact mass of 12.
- Mass number is a whole number obtained by the addition of protons and neutrons while atomic mass is the average number of protons and neutrons in an element for all of its isotopes.
- It is denoted using the letter ‘A.’
Mass Number of an element is calculated using the formula,
| Mass Number = Number of Protons + Number of Neutrons |
Check More:
Molecular Mass
[Click Here for Previous Years’ Questions]
Molecular Mass is the atomic masses of the constituent atoms that make up a compound.
- It is also known as molecular weight.
- Molecular Mass is calculated as the sum of the masses of all the atoms in a molecule.
- The obtained value is them multiplied by the number of atoms of the element.
Solved ExampleExample: Calculate the Molecular Mass of Sodium Sulphite (Na2SO3). Solution: The chemical formula of Sodium Sulphite is Na2SO3.
S and O atoms are 23 u, 32 u, and 16 u respectively. Molecular Mass of Sodium Sulphite = 2 (23) + 32 + 3(16) u = 126 u Thus, the molecular mass of Sodium Sulphite is 126 u. |
Things to Remember
- Atomic Mass is the total mass of one atom of an element.
- It is the sum total of the protons, neutrons, and electrons in an atom.
- Atomic Mass = Mass of Protons + Mass of Neutrons + Mass of Electrons
- Atomic Mass Units (amu) or Daltons are the units used for the measurement of atomic mass.
- It is also known as ‘Atomic Weight’ and is denoted by the letter ‘A’.
- Mass Number and Atomic Mass of an element are almost the same as they are the sum of the protons and neutrons of an atom.
- Atomic Number is the number of positive charges or protons in the nucleus of an atom of a given element.
Previous Years’ Questions
- One standard atomic mass unit is… (JIPMER 1998)
- The size of the nucleus of an atom of mass number… (Delhi UMET/DPMT 2009)
- The long form of Periodic Table asked on… (BHU UET 2007)
- If the atomic mass of carbon was set at 100 u…
- 1 unified atomic mass unit (1u) is equal to…
- The element, with atomic number 118 will be… (AIIMS 1995)
- The atomic number of an element M is 26R. How many electrons… (EAMCET 2003)
- If the atomic number of an element is 33, it will be placed… (NEET 1993)
- An atom of mass number A and atomic number Z… (KCET 2000)
- A nuclei X with mass number A and charge number Z… (Rajasthan PMT 2005)
Sample Questions
Ques. What is Atomic Mass? (1 Mark)
Ans. Atomic Mass is defined as the mass of a single atom in a chemical element. Atomic Mass involves the total mass of the three subatomic particles of an element namely protons, neutrons, and electrons.
Ques. What is the importance of Atomic Mass? (1 Mark)
Ans. Mass is a fundamental physical property of matter. Atomic Mass defines the total mass of an atom or a molecule. It is used to determine the average mass of atoms and molecules as well as used to solve problems related to stoichiometry.
Ques. What is the value of one amu? (1 Mark)
Ans. Atomic Mass Unit (amu) is used to measure the atomic mass of an element. 1 amu is the average of the rest mass of the proton and neutron and is equal to 1.67377 × 10-27 kg.
Ques. What is Atomic Number? (2 Marks)
Ans. The atomic number of an element is determined by how many protons are found in one of its atoms. The number of protons in each atom in an element is the same. For example, the atomic number of carbon is 6 asa ll atoms of carbon have six protons.
Atomic Number = Number of Protons in an Atom
Ques. Why is Carbon-12 selected as the reference element for calculating atomic mass? (1 Mark)
Ans. Carbon-12 was selected as the reference element for calculating atomic mass as it is the only atom with the same whole-number mass as the amu scale.
Ques. What is the difference between atomic mass and atomic weight? (1 Mark)
Ans. Atomic mass of elements is the measurement of the absolute mass of all the atomic units. Atomic weight is the generic mass of all the isotopes of the element found in a certain percentage of natural abundance.
Ques. List the first 10 elements from the periodic table with their atomic masses. (3 Mark)
Ans. Atomic masses of the first 10 elements of the periodic table are given below:
| Atomic Number | Element | Atomic Mass |
|---|---|---|
| 1 | Hydrogen | 1.008 |
| 2 | Helium | 4.0026 |
| 3 | Lithium | 6.94 |
| 4 | Beryllium | 9.0122 |
| 5 | Boron | 10.81 |
| 6 | Carbon | 12.011 |
| 7 | Nitrogen | 14.007 |
| 8 | Oxygen | 15.999 |
| 9 | Fluorine | 18.998 |
| 10 | Neon | 20.18 |
Ques. Can Atomic Mass and Atomic Weight Ever Be the Same? (2 Marks)
Ans. The atomic mass and atomic weight of an element that only has one isotope will be the same. When working with a single isotope of an element, atomic mass, and atomic weight may also equal each other. In this situation, you calculate using the atomic mass of the element rather than the atomic weight from the periodic table.
Ques. How can you measure Atomic Weight? (1 Mark)
Ans. Atomic weight is the generic mass of all the isotopes of the element found in a certain percentage of natural abundance.
Ques. If the number of electrons in an atom is 8 and the number of protons is also 8, then
(i) What is the atomic number of the atom?
(ii) What is the charge on the atom? (2 Marks)
Ans. (i) Atomic number = Number of protons = 8
(ii) In the given atom, the total number of positive charges is equal to the total number of negative charges.
Number of protons (8) = Number of electrons (8)
So, the charge on the atom will be zero.
Ques. What is the mass of hydrogen in terms of AMU?
a) 1.0020 amu
b) 1.0070 amu
c) 1.0180 amu
d) 1.0080 amu (2 Marks)
Ans. d) 1.0080 amu
Explanation: The mass of a hydrogen atom is 1.6736×10-24 g. When it is converted in terms of amu, 1.6736×10-24g should be divided by 1.66056×10-24 g.
1.6736×10-24 g/1.66056×10-24 g = 1.0078 amu = 1.008 amu. This is the process to measure any atomic mass in amu.
Ques. “Amu” nowadays is replaced by ____ :
a) am
b) kg
c) g
d) u (2 Marks)
Ans. a) u
Explanation: “Amu” nowadays has been replaced by “u”. The Atomic Mass Unit used to be “amu,” which was changed to “u” and is now known as Unified Mass. The mass of one nucleon is one unified atomic mass unit, which is also equivalent to 1 g/mol.
Ques. ______ is the sum of atomic masses of the elements present in a molecule:
a) Average Atomic Mass
b) Atomic Mass
c) Gram Formula Mass
d) Molecular Mass (2 Marks)
Ans. d) Molecular Mass
Explanation: The total atomic masses of the atoms in a molecule is known as molecular mass. The amount of a substance with the same exact mass in grams as the formula mass in amu is called gram formula mass. The “amu” is the standard unit of mass for measuring mass on a molecular or atomic scale.
Check Out:





Comments